Equilibrium Constants (Keq) Explained
advertisement

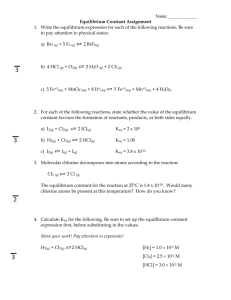
EQUILIBRIUM CONSTANTS (Keq) One way to predict which direction is favored in a reversible chemical reaction is to look at the concentrations of the reactants compared to the concentrations of the products. Two Norwegian chemists, Cato Maximilian Guldberg and Peter Waage proposed the Law of Mass Action as a general description of the equilibrium condition. Guldberg and Waage mathematically compared the ratio of products to reactants and took into consideration the coefficients of each in the balanced chemical equation. The following generic equation will be used to show how they set up their mass action expression (also called the equilibrium expression). mA + nB <---------------> p C + qD In this reaction A and B represent the reactants and C and D represent the products. The small letters in italics represent the coefficients in the balanced equation. The mass-action expression is shown by multiplying the concentrations of the products times each other and placing them over the reactants times each other. The coefficients become exponents in the massaction expression. The mass-action expression for this reaction is shown below. [C]p x [D]q -------------[A]m x [B]n If the concentrations of the reactants and products are known, they can be inserted into the mass-action expression and the equilibrium constant (Keq) can be calculated. Concentration for calculating equilibrium constants is usually in moles per liter (M). A large Keq tells us that the forward direction is favored in a reaction and a small Keq tells us that the reverse direction is favored. A large Keq is usually considered to be a number larger than 1 and a small Keq is usually considered to be a number smaller than 1. The following example will show how to set up the mass-action expression for a reaction and how to calculate the Keq when the concentrations are known at equilibrium. Example 1 Find the Keq for the following reaction and predict if the forward or reverse direction is favored. At equilibrium, the following concentrations were measured. [HI] = 1.544M [H2] = 0.228M [I2] = 0.228M H2(g) + I2(g) <---------------------> 2HI(g) The mass-action expression for this reaction would be: [HI]2 -----------[H2] x [I2] To calculate the equilibrium constant we plug in the equilibrium concentrations that were given in the problem and do the math. [1.544]2 Keq = ------------------[0.228] [0.228] = 45.9 The relatively large Keq tells us that the forward direction is favored at equilibrium. If you know the Keq for a reaction you can calculate what the equilibrium concentrations would be for the reactants and products. The following example shows how this can be done. Example 2 Find the equilibrium concentration of N2 given the following information. Keq = 1.20 x 10-2 [H2] = 1.25M [NH3] = .450M 3H2(g) + N2(g) <---------------> 2NH3(g) [NH3]2 Keq = 1.20 x 10-2 = ------------------[H2]3 [N2] [.450]2 [N2] = ----------------------1.20 x 10-2 [1.25]3 = = [.450]2 ------------------[1.25]3 [N2] 8.64 M Example 3 In a 1.00 Liter container, 1 mole of H2O is decomposed according to the equation 2H2O(g) <-----------------------> 2H2(g) + 02(g) At the equilibrium point, 0.125 moles of O2 are present. Calculate the concentrations of H2 and H20 in moles per liter and calculate the equilibrium constant. Since the H2 and O2 form in a 2:1 ratio (from the balanced equation), if there are .125 moles of O2, there should be twice as much of the H2, or 2.50 moles. We can figure out the number of moles of H2O left by applying the same logic. For every 2 moles of H2 that form, 2 moles of H2O must react, so if we have .250 moles of H2 then .250 moles of H2O must have reacted. Since we started with 1 mole of H2O and .250 moles have reacted, then .750 moles of H2O must remain in the container. The number of moles of each of the substances will be the same as their concentrations in moles per liter since they are in a 1.00 L container. [O2] = .125M [H2] = .250M [H2O] = .750M Now plug the concentrations into the mass-action expression for the reaction and the equilibrium constant can be calculated. [H2]2 [O2] Keq = ------------------[H2O]2 = [.250]2 [.125] -------------------[.750]2 = 0.0139