BIO.3
advertisement

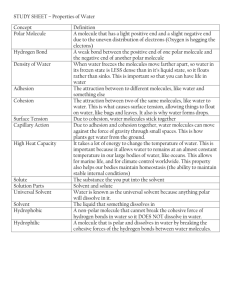
BIO.3 The student will investigate and understand the chemical and biochemical principles essential for life. Key concepts include a) water chemistry and its impact on life processes Water is a dipole. Within the molecule, electrons are drawn more strongly to the single oxygen atom, thus exposing some positive charge on the two hydrogen atoms and creating some excess negative charge on the oxygen end of the molecule. Thus one end of the molecule tends to be positive and the other negative. It is this dipole that gives water its unique properties: high heat capacity, cohesion, adhesion, and ability to dissolve most substances to some extent. Water has a high heat capacity. This means that it can absorb large amounts of heat without becoming hot and release large amounts of heat without becoming cold. So, water moderates and stabilizes temperature, a desirable property as a medium for life processes. It comprises as much as 70% of the mass of living organisms. Because positive and negative charges are drawn to each other, water forms hydrogen bonds with other water molecules (hydrogen of one molecule is drawn to the oxygen of another molecule). This is referred to as cohesion. It is responsible or the surface tension of water. At an interface with air, the surface of water has some strength and will not break easily. It beads up and will support the weight of some aquatic insects like water striders. Some fun activities to demonstrate this in class include seeing how many drops of water can be placed on a penny and floating small paper clips on the surface of a glass of water. The surfaces of many substances are charged, and water will be drawn to such surfaces. This is referred to as adhesion. It is responsible for capillary action, the means by which water moves up into a plant; the attraction of the water molecules to the walls of the tube is stronger than the pull of gravity on the water. This can be demonstrated in class by using paper towels or chromatography paper in which the edge is immersed in water. Another simple demonstration of adhesion involves placing 1 mL of distilled water on a piece of wax paper (no charge) and another on a piece of aluminum foil (charged). Measure the diameter of the water droplet, it will be much larger on the foil. This is also a good activity to do mean, median, and mode on class data. Water is the universal solvent. If a substance is also polar, it will dissolve in water. If a substance such as sodium has a positive charge, the negative end of water molecules will surround an ion, forming a hydration sphere and shielding it from bonding with a negatively charged substance. If the substance has a negative charge, like chlorine, the positive end of the water molecules will surround it. Because lipids (fats) are not polar (hydrophobic) they will form droplets in water and will separate. This is an important property in cells, because lipids form cell organelle structures. In a given amount of water, some of the water molecules will break down into hydrogen ions (H+) and hydroxide (OH‾) ions. In some solutions, either the hydrogen ion or the hydroxide ion will be chemically or biologically removed. When they hydrogen ion equals the hydroxide ion the solution is neutral. If there is more hydrogen, the solution is acidic and if there is more hydroxide, it is basic. pH is the negative log of the hydrogen ion concentration. 0< pH < 7 is acidic, 7 is neutral, 7< pH < 14 is basic. Biochemical processes often require a specific pH to occur. b) the structure and function of macromolecules Please note that learning this material early in a course is very difficult and abstract to students. I follow the practice of introducing a macromolecule and its function as it is needed in the remainder of the content areas. There are five common elements that make up most organic macromolecules: carbon, hydrogen, oxygen, nitrogen, and phosphorous. Carbon makes up the backbone of all macromolecules. It must form four bonds, some of these bonds may be with other carbon atoms in double or triple bonds. Most of the time it forms bonds with hydrogen, which can only form one bond. There are four major groups of macromolecules: carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates are made of carbon, hydrogen, and oxygen in the ratio of 1:2:1. These are the sources of energy in food. Monosaccharides are simple sugars like glucose and fructose and are the energy used by cells. Table sugar, sucrose, is a disaccharide, made up of a molecule of fructose bonded to a molecule of glucose. Polysaccharides such as starch and glycogen are chains of sugars that are a means of storing energy in cells. Lipids (fats, sterioids, phospholipids, waxes). They may be saturated or unsaturated (multiple bonds between carbons); the carbon to hydrogen bond in lipids contains a lot of energy. Lipids and phospholipids make up cell membranes; cholesterol is an important component of animal cell membranes; chlorophyll and other pigments are lipids that can absorb light for photosynthesis. Proteins are chains of amino acids. Amino acids are named for the amine groups which contain nitrogen. There are twenty different amino acids. Some amino acids are charged, some are polar, and some are non-polar; thus, a long chain of amino acids will fold back on itself in secondary and tertiary three dimensional structures. This structure is important in the functioning of enzymes. An enzyme is a protein that acts as a catalyst in promoting chemical reactions within the cell. Other proteins are structural, making up skin, hair, tendons, etc. Proteins cause muscle contraction and hemoglobin carries oxygen to your muscles. Proteins are the direct expression of genes. Nucleic acids are chains of nucleotides. Each nucleotide consists of a 5 carbon sugar (ribose or deoxyribose) bonded to a phosphate (carbon 5) and one of five nitrogenous bases bonded to carbon 1 (adenine, thymine, cytosine, guanine, uracil). If the sugar backbone is deoxyribose, then the first four bases are used and the nucleic acid is DNA – deoxyribonucleic acid. If the sugar backbone is ribose, then uracil is substituted for thymine, and the nucleic acid is RNA – ribonucleic acid. Nucleic acids are generally double helixes as discussed earlier, however, some forms of life have single strands of DNA and RNA is often single stranded. Although not really a macromolecule, the energy currency of all cells is ATP, adenosine triphosphate. The breakdown of carbohydrates in cellular metabolism converts energy to ATP (three phosphate groups linked) which carries energy to different places within the cell. Energy is released when enzymes break off one of the phosphate groups from this molecule. c) the nature of enzymes; As discussed above, enzymes are complexly folded proteins which catalyze reactions within cells. This is accomplished by reducing the activation energy hurdle for the reaction. They can bond to a complex molecule and cause it to break down or they can bring two molecules into proximity and cause them to bond to each other. Each enzyme has a specific function which is determined by the way it is folded to create an activation site into which the substrate just fits. This can actually change the configuration of the enzyme temporarily so that the catalyzed reaction proceeds. When the product(s) of the reaction are released, the enzyme returns to its original configuration and catalyzes another reaction. Each enzyme will function best at a specific temperature, pH, and solution concentration. This can be different for different organisms even though the reaction is the same. For example, enzymes functioning in respiration in deep sea fish will function best at very cold temperatures. The same reaction in mammals occurs most efficiently at much warmer temperatures. d) and the capture, storage, transformation, and flow of energy through the processes of photosynthesis and respiration. Photosynthesis and cellular respiration (not breathing) are opposite processes. Photosynthesis captures energy in the form of light and stores this energy in simple sugars, namely glucose. Cellular respiration breaks down glucose and stores the energy in the form of ATP to power cellular processes. All cells have some form of cellular respiration, however, not all cells can harvest energy from the environment through photosynthesis or chemosynthesis. These last two processes are carried out by primary producers in ecosystems. The stored energy is passed to other organisms through the food chain, a very inefficient process (usually about 10% efficiency). Photosynthetic organisms contain specialized lipids called pigments that are found in the membranes of plastids or chloroplasts and in the cell membranes of some prokaryotes. The generalized equation for photosynthesis is 6CO2 + 6H2O + sunlight → C6H12O6 + 6O2 or 6 molelcules of carbon dioxide gas, plus 6 molecules of water in the presence of sunlight yields one molecule of glucose and 6 molecules of oxygen gas. In light dependent reactions, light is absorbed by pigments and stored as energy in ATP and NADPH. In the processs, oxygen is removed by enzymes from water and released as a byproduct. In general, only red light and blue light are used by chlorophyll. Carotenoids use some blue-green light waves. In dark reactions, the Calvin cycle fixes carbon in 3 carbon sugars using carbon dioxide and the stored energy from ATP and NADPH. All these reactions are mediated by enzymes. Cellular respiration is the reverse process and can be either anaerobic (in absence of oxygen) or aerobic (in presence of oxygen). This is the generalized reaction for cellular respiration: C6H12O6 + 6O2 → 6CO2 + 6H2O + energy (ATP) Glucose and oxygen in the presence of required enzymes yields cabon dioxide, water and energy stored in ATP. Note that this is the reverse of the photosynthesis reaction. Cellular respiration occurs in two stages in different parts of the cell. In stage one, glycolysis occurs in the cytoplasm. In this process glucose is broken down into two pyruvate molecules (producing a gain of 2 ATP molecules and 2 NADPH molecules). Stage 2 occurs in the mitochondria and is referred to as the Krebs Cycle. During this cycle, pyruvate is broken down and carbon dioxide is released. Energy is stored in ATP (gain of 37 ATP molecules), oxygen is combined with hydrogen to form water as a byproduct. Cellular respiration in the absence of oxygen is of two kinds: lactic acid fermentation and alcohol fermentation. During intense exercise, cells may be deprived temporarily of oxygen. In this case, pyruvate is converted to lactate and ATP. Lactate can build up in muscle cells causing soreness and fatigue. In some organisms such as yeast, alcohol fermentation occurs and pyruvate is converted to ethanol, releasing carbon dioxide and producing ATP. This is an important process in some food production such as bread, beer and wine. Photosynthesis and cellular respiration are summarized in the diagram below. Remember, all reactions are mediated by enzymes and occur in specific places within the cell. Note that the drawing is not a cycle. As is presented in a later SOL BIO.9, nutrients recycle (matter), but energy does not, so I have not closed the cycle. O2 CO2 H2O ATP NADPH pigments Calvin Cycle Light dependent Glucose Glycolysis O2 H2O ATP Krebs Cycle Pyruvate & ATP CO2 After reviewing material in your textbook, go to the file labeled BIO.3 Review Response and open it in Word. Type your answers below each question and make them a distinctive readable color or font. E-mail this file as an attachment