section7and8
advertisement

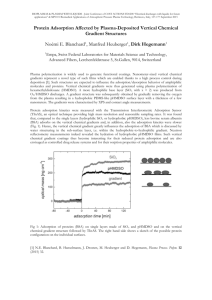
7. Chemical Modification for Nonspecific Adsorption Nonspecific adsorption of biomolecules is simple and moderate. For WGMB applications, microspheres acts as the solid support. Plasma cleaned spheres introduce surface hydroxyl groups which interact with methoxy/ethoxy components of the silanes. For our nonspecific adsorption experiments, we use 3-aminopropyltriethoxysilane1 (APTES) leaving a rug of amines as reactive groups (Fig.9). We also use a modified technique for vapor phase deposition in a vacuum chamber2. Briefly, 1 mL of fresh APTES is placed into a vacuum chamber, refluxed briefly, slowly pressurized, and cured in a hot oven at 120° C. NH2 carries a pKa of about 8, meaning at our pH buffer (PBS 7.4), it takes on a proton becoming NH3+, highly attractive to negatively charged molecules. Fig. 9. Amino-silanization using APTES on cleaned microsphere support. This type of modification was used for all nonspecific binding experiments which will be discussed next. 8. Molecular and Artificial Examples: BSA, Antibody IgG, Polystyrene Spheres BSA is an abundant protein found in the serum of bovine having a molecular weight of 66,000 g/mol. A BSA layer on a counter-charged glass surface has a thickness of about 3 nm3,4. Binding experiments were first conducted to demonstrate the ability of the instrument to sense small-molecule protein adsorption5 and to correlate the measurements with thickness estimation based on theory mentioned earlier. With a pI of 4.7, BSA has a net negative charge in pH 7.4 buffer. Therefore, our amino-silanized spheres are attractive to these biomolecules. 20 μl of a 50 μM solution of BSA in 10 mM phosphate buffered saline was mixed with 980 ul of PBS buffer bringing the final concentration of protein to 1 M. Sample was continuously stirred and resonant dip traces were tracked (Fig. 10). Complete saturation was observed in ~35 seconds. Fig. 10 BSA adsorption. Resonance dip trace from 1310-nm laser recorded over 5 minutes. In equation (25) we relate the wavelength shift of binding events to thickness of a monolayer. We measure a thickness of ~2.1 nm indicating a surface coverage of xxx. (discuss calculation with Dr. Arnold). Antibody IgG too is a protein with a molecular weight of 155,000 g/mol, about 2.5 times the size of BSA. It has a pI of around 6.8 so similar buffer conditions and surface modification methods were used. At a 62 nM concentration, the resultant dip trace is given in Fig.11. Normalized shift gives us a thickness of 4.6 nm, approximately 2.5 times the shift we observed with our BSA experiments. Fig.11 Dip trace of antibody IgG nonspecific adsorption onto amino-silanized sphere. Simulation of Viral Binding with Polystyrene Spheres We then moved onto larger molecules which would more closely simulate large virion detection, such as HIV. We looked at polystyrene spheres of diameter 100 nm. The polystyrene beads were carboxylated, thus amino-silanization methods were again employed. Polystyrene beads were injected into a 1cm3 cuvette with constant stirring. Complete saturation was observed at ~3 hours after injection. Compared to the shift resulting from BSA adsorption, we estimated a shift of ~60 nm for a 100 nm particle. We measure a thickness of approximately 45 nm (Fig.12) which agrees with theory predicted (Dr. Teraoka’s analysis). (answer, does it fit theory, what did you expect?) Fig. 12. Binding of polystyrene nanoparticles to sphere surface and resonant shift associated with binding events. Inset, comparison of shift between BSA and IgG. We will next discuss virus sensing using WGM and present results on nonspecific virus adsorption experiments. We follow with a modified surface treatment protocol and incorporation of microfluidic capabilities to our system for specificity detection purposes. 1 N. Zammatteo, S. Hamels, F. De Longueville, I. Alexandre, J. L. Gala, F. Brasseur, and J. Remacle, "New chips for molecular biology and diagnostics," Biotechnol Annu Rev 8, 85-101 (2002). K. H. Choi, J. P. Bouroin, S. Auvray, D. Esteve, G. S. Duesberg, S. Roth, M. Burghard, “Controlled deposition of carbon nanotubes on a patterned substrate,” Surf Sci 462, 1952002 (2000). 2 3 T. J. Su, J. R. Lu, R. K. Thomas, Z. F. Cui, J. Penfold, "The conformational Structure of Bovine Serum Albumin Layers Adsorbed at the Silica-Water Interface," J Phys Chem B 102, 8100-8108 (1998). 4 T. J. Su, J. R. Lu, R. K. Thomas, Z. F. Cui, "Effect of pH on the Adsorption of Bovine Serum Albumin at the Silica/Water Interface Studied by Neutron Reflection," J Phys Chem B 103, 3727-3736 (1999). 5 F. Vollmer, D. Braun, and A. Libchaber, "Protein detection by optical shift of a resonant microcavity," Appl. Phys. Lett 80, 4057-4059 (2002).