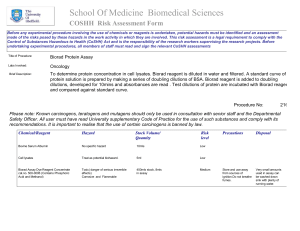
Quantification of Proteins for Western Blot
advertisement
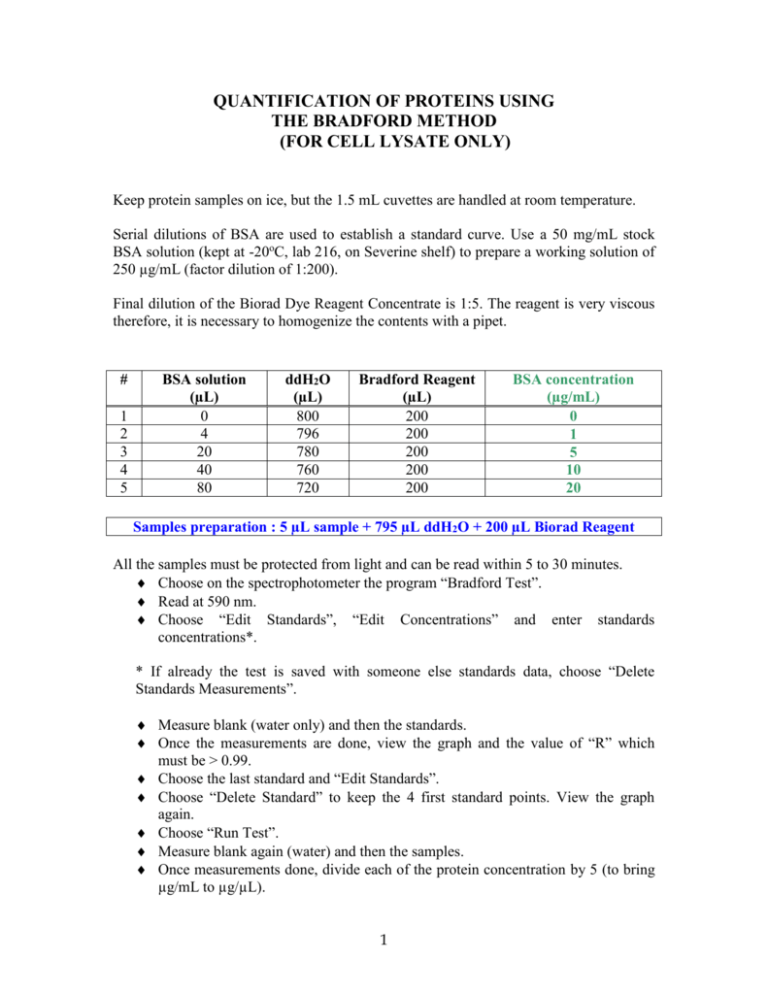
QUANTIFICATION OF PROTEINS USING THE BRADFORD METHOD (FOR CELL LYSATE ONLY) Keep protein samples on ice, but the 1.5 mL cuvettes are handled at room temperature. Serial dilutions of BSA are used to establish a standard curve. Use a 50 mg/mL stock BSA solution (kept at -20oC, lab 216, on Severine shelf) to prepare a working solution of 250 µg/mL (factor dilution of 1:200). Final dilution of the Biorad Dye Reagent Concentrate is 1:5. The reagent is very viscous therefore, it is necessary to homogenize the contents with a pipet. # 1 2 3 4 5 BSA solution (µL) 0 4 20 40 80 ddH2O (µL) 800 796 780 760 720 Bradford Reagent (µL) 200 200 200 200 200 BSA concentration (µg/mL) 0 1 5 10 20 Samples preparation : 5 µL sample + 795 µL ddH2O + 200 µL Biorad Reagent All the samples must be protected from light and can be read within 5 to 30 minutes. Choose on the spectrophotometer the program “Bradford Test”. Read at 590 nm. Choose “Edit Standards”, “Edit Concentrations” and enter standards concentrations*. * If already the test is saved with someone else standards data, choose “Delete Standards Measurements”. Measure blank (water only) and then the standards. Once the measurements are done, view the graph and the value of “R” which must be > 0.99. Choose the last standard and “Edit Standards”. Choose “Delete Standard” to keep the 4 first standard points. View the graph again. Choose “Run Test”. Measure blank again (water) and then the samples. Once measurements done, divide each of the protein concentration by 5 (to bring µg/mL to µg/µL). 1