Reaction Types: Synthesis
advertisement

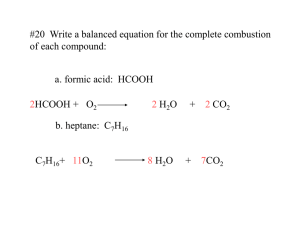
Reaction Types: Synthesis Important notes to remember: (1) NONE of the equations are balanced!! and (2) make sure to write correct formulas. DO NOT just copy the subscripts from the reactants over into the products. Synthesis are, at this introductory level, almost always the reverse of a decomposition reaction. That means that two pieces join together to produce one, more complex compound. These pieces can be elements or simpler compounds. Complex simply means that the product compound has more atoms than the reactant molecules. Usually!! Written using generic symbols, it is usually shown as: A + B ---> AB These are some examples: Mg + O2 ---> MgO H2 + O2 ---> H2O K + Cl2 ---> KCl Fe + O2 ---> Fe2O3 Notice that two elements are combining in each example. Synthesis can also be two compounds making a more complex compound (or a compound and an element joining together) as in these examples: CaO + CO2 ---> CaCO3 Na2O + CO2 ---> Na2CO3 KCl + O2 ---> KClO3 Ba(ClO3)2 ---> BaCl2 + O2 Notice how, in every case so far, there is only one substance on the right-hand (product) side. This is not always the case in a synthesis reaction. Sometimes there will be two products. Here's an example: CO2 + H2O ---> C6H12O6 + O2 You might recognize this as the photosynthesis equation. However, the majority of what you will see at this level will be two substances combining to make one product. Here's another example of a synthesis reaction: H2 + O2 ---> H2O2 This happens to be a reaction that can never take place. Hydrogen peroxide is made in other ways, NOT by direct union of the elements. Nonetheless, it is a valid synthesis reaction and useful in contexts other than how H2O2 is made. Since synthesis reactions are the reverse of decomposition, you might ask if the decomp. categories apply, just in reverse. The answer is yes! 1) Direct union of two elements will produce a binary compound. 2) Metallic oxides and carbon dioxide react to produce carbonates. 3) Binary salts and oxygen react to produce a chlorate. Here is one more category of decomposition reactions: CaO + H2O ---> Ca(OH)2 Na2O + H2O ---> NaOH N2O5 + H2O ---> HNO3 P2O5 + H2O ---> H3PO4 The first two substances are metallic oxides and the last two are nonmetallic oxides. In each case, the oxide plus water will produce a base (in the case of the metallic oxide) or an acid (in the case of the nonmetallic oxide). Here is one example of each category which are then solved below: 1) LiCl + O2 ---> 2) Na2O + 3) SO3 + 4) N2 + CO2 ---> H2O ---> H2 ---> Reaction Types: Decomposition Important notes to remember: (1) NONE of the equations are balanced!! and (2) make sure to write correct formulas. DO NOT just copy the subscripts from the reactants over into the products. During decomposition, one compound splits apart into two (or more pieces). These pieces can be elements or simpler compounds Written using generic symbols, it is usually shown as: AB ---> A + B However, that really only works for splitting apart into the elements, like these examples. HgO ---> Hg + O2 H2O ---> H2 + O2 MgCl2 ---> Mg + Cl2 FeS ---> Fe + S Decomposition can also split one compound into two simpler compounds (or compound and an element) as in these examples: CaCO3 ---> CaO + CO2 Na2CO3 ---> Na2O + CO2 KClO3 ---> KCl + O2 Ba(ClO3)2 ---> BaCl2 + O2 Notice how, in every case so far, there is only one substance on the left-hand (reactant) side. This is always the case in a decomposition reaction. Don't forget that!! Figuring out what the products are in decomposition is harder (maybe you'll think it's easier!!) because you will have to recognize several categories of decomposition reactions. Here are your first (yes, there's more!) three: 1) All binary compounds (like the four in the first example set above) will break down into their elements. 2) All carbonates (like the first two in the second example set above) break down to the oxide and carbon dioxide. 3) Chlorates (like KClO3 and Ba(ClO3)2 in the example) will break down to the binary salt and oxygen. Here is one more category of decomposition reactions: Ca(OH)2 ---> CaO + H2O NaOH ---> Na2O + H2O HNO3 ---> N2O5 + H2O H3PO4 ---> P2O5 + H2O The first two substances are bases and the last two are acids. In each case, the acid or base breaks down into the oxide of the metal (in the case of bases) or the oxide of the nonmetal (in the case of acids) plus water. Here is one example of each category which are then solved below: Decomposition 1) NaClO3 ---> 2) Li2CO3 ---> 3) KOH ---> 4) NaCl ---> Reaction Types: Combustion Important notes to remember: (1) NONE of the equations are balanced!! and (2) make sure to write correct formulas. DO NOT just copy the subscripts from the reactants over into the products. Combustion, at its most general, can mean the reaction of oxygen gas (O2) with anything. However, we will understand combustion to mean the reaction of oxygen with an compound containing carbon and hydrogen. A common synonym for combustion is burn. Written using generic symbols, it is usually shown as: CxHy + O2 ---> CO2 + H2O These are some examples: CH4 + O2 ---> CO2 + H2O C2H6 + O2 ---> CO2 + H2O C6H12O6 + O2 ---> CO2 + H2O C2H5OH + O2 ---> CO2 + H2O Notice that some compounds contain carbon, hydrogen AND oxygen. However, the products are all the same, in every reaction. Isn't that great? We could vary it a bit by adding nitrogen (burns to form NO 2) to the compound formula or sulfur (burns to form SO2). Like this: C21H24N2O4 + O2 ---> CO2 + H2O + NO2 C2H5SH + O2 ---> CO2 + H2O + SO2 Here are some combustions: Combustion 1) C7H6O + O2 ---> 2) CH3COCH3 + 3) H2C2O4 + O2 ---> O2 ---> Reaction Types: Double Replacement Important notes to remember: (1) NONE of the equations are balanced!! and (2) make sure to write correct formulas. DO NOT just copy the subscripts from the reactants over into the products. During double replacement, the cations and anions of two different compounds switch places. Written using generic symbols, it is: AB + XY ---> AY + XB A and X are the cations (postively-charged ions) in this example, with B and Y being the anions (negatively-charged ions). Here is another way to look at the above generic example: a) the outside portions (the cation A and anion Y) combine to make a formula called AY. b) The inside portions (the anion B and the cation X) switch order so that X (postively charged) goes first and B (negatively charged) goes second making a formula called XB. Keep in mind that, when it comes to writing actual formulas, you MUST write chemically correct formulas. Please do not assume from the AY and XB examples that the product formulas will always be one-to-one in terms of positive and negative. Some examples of actual reactions are: KOH + H2SO4 ---> K2SO4 + H2O FeS + HCl ---> FeCl2 + H2S NaCl + H2SO4 ---> Na2SO4 + HCl AgNO3 + NaCl ---> AgCl + NaNO3 Note that none of them are balanced yet. These three are also examples of double replacement, but there is something special about them: CaCO3 + HCl ---> CaCl2 + CO2 + H2O K2SO3 + HNO3 ---> KNO3 + SO2 + H2O NH4Cl + NaOH ---> NaCl + NH3 + H2O Notice how, one of the two product compounds decomposes. Whenever H2CO3, H2SO3, or NH4OH is a product formula, the correct technique is to write the products as done in the examples. Don't forget that!! In other words, CaCO3 + HCl ---> CaCl2 + H2CO3 is incorrect. One additional comment on the above - a acid or a base is one of the two substances involved in the reactants. If no acid or base, the decomposition does not take place. See practice problem #6 for an example. There is another example in the 10 problems, but you'll have to figure out which one!! In double replacement, both reactants are compounds, each with a cation part and an anion part. Diatomic elements do not count; they are included in the single replacement category. Typically, you will be given the left-hand (reactant side) and asked to provide the products to the reaction. You need to be able to recognize double replacement reactions AND be able to break a formula apart into proper cations and anions as well as write correct formulas Here are several examples which are solved below: 1) Ca(OH)2 + HCl ---> 2) Al(NO3)3 + H2SO4 ---> 3) Pb(NO3)2 + K2S ---> 4) Pb(NO3)2 + CuSO4 ---> Reaction Types: Single Replacement Important notes to remember: (1) NONE of the equations are balanced!! and (2) make sure to write correct formulas. DO NOT just copy the subscripts from the reactants over into the products. During single replacement, one element replaces another element in a compound. There are two different possibilities: 1. One cation replaces another. Written using generic symbols, it is: AX + Y ---> YX + A Element Y has replaced A (in the compound AX) to form a new compound YX and the free element A. Remember that A and Y are both cations (postively-charged ions) in this example. Some examples are: Cu + AgNO3 ---> Ag + Cu(NO3)2 Fe + Cu(NO3)2 ---> Fe(NO3)2 + Cu Ca + H2O ---> Ca(OH)2 + H2 Zn + HCl ---> ZnCl2 + H2 Notice how, when hydrogen gets displaced, I write it as a diatomic. I do that because elemental hydrogen is diatomic. Don't forget that!! 2. One anion replaces another. Written using generic symbols, it is: A + XY ---> XA + Y Element A has replaced Y (in the compound XY) to form a new compound XA and the free element Y. Remember that A and Y are both anions (negatively-charged ions) in this example. The only examples the ChemTeam knows about involve halogens, so here are two examples: Cl2 + NaBr ---> NaCl + Br2 Br2 + KI ---> KBr + I2 In single replacement, one reactant is always an element. It does not matter if the element is written first or second on the reactant side. The other reactant will be a compound. Typically, you will be given the left-hand (reactant side) and asked to provide the products to the reaction. You need to be able to recognize single replacement reactions AND be able to break a formula apart into proper cations and anions as well as write correct formulas Here are several examples which are solved below: 1) ZnS + O2 ---> 2) K+ 3) Fe + HCl ---> 4) NaI + Br2 ---> H2O --->