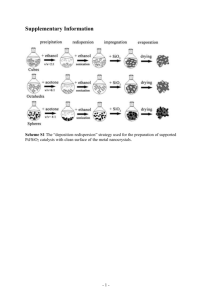
Supplementary data One-pot synthesis of core
advertisement

Supplementary data One-pot synthesis of core-shell Cu@SiO2 nanospheres and their catalysis for hydrolytic dehydrogenation of ammonia borane and hydrazine borane Qilu Yao,1 Zhang-Hui Lu,*1 Zhujun Zhang,1 Xiangshu Chen,*1 & Yaqian Lan2 1 Jiangxi Inorganic Membrane Materials Engineering Research Centre, College of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang 330022, China, 2Jiangsu Key Laboratory of Biofunctional Materials, College of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, China E-mail address: luzh@jxnu.edu.cn (Z.-H. Lu), cxs66cn@jxnu.edu.cn (X. Chen). S1 Synthesis of hydrazine borane (N2H4BH3, HB). Hydrazine borane was synthesized by the method reported in literature.60 Briefly, 21.42 g hydrazine hemisulfate salt ((H4N2)2H2SO4) and 10 g sodium borohydride (NaBH4) were suspended in 80 mL of anhydrous dioxane, and kept stirring for 2 days under Ar atmosphere. The mixture was separated by centrifugation at 12000 rpm for 12 min. Then, the solvent was removed under vacuum at 313 K overnight and n-pentene was added to precipitate HB. Finally, the precipitate was separated by centrifugation, washed with n-pentene for several times, and dried under vacuum at 313 K. The obtained material is a white solid, and its purity verified by 1H NMR and XRD. 1H NMR (δ/ppm, probe head Dual 1 H/13C, 300.13 MHz, CD3CN, 30 ºC, J/Hz): 5.45 (s, 2H, B-NH2), 3.44 (s, 2H, N-NH2), 1.42(q, 3H, 1JHB=95 Hz) in agreement with the literature values. Figure S1 | (a) Powder X-ray diffraction pattern and (b) solid-state 1H NMR spectrum of the obtained HB. S2 Figure S2 | The corresponding EDX spectrum of the Cu@SiO2 core-shell nanospheres. S3 Figure S3 | Cu 2p XPS spectra for the Cu@SiO2 core-shell nanospheres. S4 Figure S4 | Nitrogen adsorption-desorption isotherms of the Cu/Commercial SiO2 NPs. S5 Figure S5 | DFT pore size distribution of the Cu@SiO2 core-shell nanospheres. S6 Figure S6 | CO-TPD curves obtained on the (a) Cu@SiO2 and (b) Cu/Commercial SiO2 NPs. S7 Table S1 TOF values of Cu nanocatalysts tested in hydrogen generation from the hydrolysis of AB at room temperature. Catalyst TOF (mol H2 mol Cu-1 min-1) Ref. Cu/RGO 3.61 37 Cu@SiO2 3.24 This work Flower-like Cu* 2.41 41 Zeolite-confined Cu 1.25 35 p(AMPS)-Cu 0.72 57 Cu/γ-Al2O3 0.27 54 CuCl2 0.23 40 Cu2O NPs 0.18 34 Cu NPs 0.06 34 Note: *Methanolysis of AB S8 Figure S7 | (a) Hydrogen generation from the hydrolysis of HB (0.2 M, 5 mL) by the Cu@SiO2 core-shell nanospheres at 298-323 K (Cu/HB = 0.09). (b) The Arrhenius plot (ln k vs. 1/T). S9 Figure S8 | Percent of initial catalytic activity retained in the successive runs for the hydrolysis of AB and HB in the presence of Cu/Commercial SiO2 at 298 K (Cu/(AB or HB) = 0.09). S10 Figure S9 | Representative TEM images of the Cu@SiO2 core-shell nanospheres after catalytic reaction. S11 Figure S10 | Hydrogen generation from the hydrolysis of AB (0.2 M, 5 mL) in the presence of Cu@SiO2 and Cu@SiO2-573 K at 298 K (Cu/AB = 0.09). S12 Figure S11 | Hydrogen generation from the hydrolysis of HB (0.2 M, 5 mL) in the presence of Cu@SiO2 and Cu@SiO2-573K at 298 K (Cu/HB = 0.09). S13