Photolysis of Atmospherically Relevant Carbonyl Compounds in
advertisement

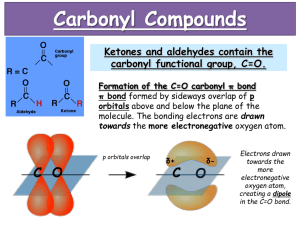
Photolysis of Atmospherically Relevant Carbonyl Compounds in Different Solutions Stephanie H. Kim, Claire R. Engelmann Mentor: Sergey Nizkorodov Carbonyl compounds in the atmosphere, namely aldehydes and ketones, are involved in the formation of tropospheric ozone and photochemical smog, and account for much of the chemical reactivity and toxicity of secondary organic aerosols (SOA) and primary organic aerosols (POA). Therefore, it is important to study their formation and removal mechanisms to better understand their active roles in atmospheric processes. Carbonyl compounds are also known to readily photodegrade in the presence of solar radiation to give rise to secondary products. However, condensed-phase photolysis of these carbonyl compounds in water and in organic matrices (particulate matter) has not been extensively studied. (1R)-(+)-camphor and 1,2cyclohexanedione were selected to model the gas, liquid, and solid-phase photolysis of atmospherically relevant carbonyl compounds using broadband 300–400 nm and 254 nm UV radiation. Solutions of carbonyls were prepared in various organic solvents, then photolyzed in the gas or liquid-phase using a Teflon bag or filtered lamp setup, respectively. Solid carbonyl compounds were photolyzed at different temperatures using a custom-made photolysis setup. The gas-phase products were analyzed by proton transfer reactionmass spectrometry (PTR-MS), while the liquid and solid-phase products were analyzed by high performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GCMS). Extinction coefficients of (1R)-(+)-camphor and 1,2-cyclohexanedione were also experimentally derived using UV-Vis analysis. The results revealed that camphor was highly resilient to photolysis compared to the fast photodegradation of 1,2cyclohexanedione; however, preliminary data indicated product formation from both compounds. Further analysis will be done to identify these products.