Ecology MAI key concepts
advertisement
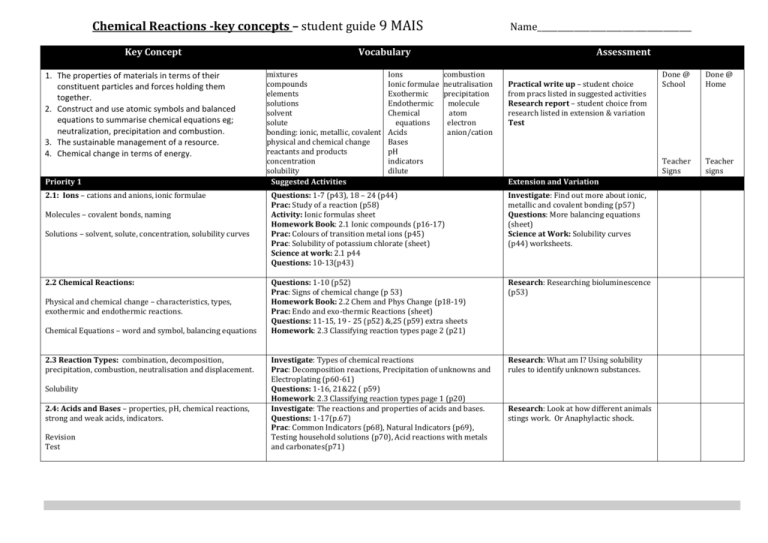
Chemical Reactions -key concepts – student guide 9 MAIS Key Concept 1. The properties of materials in terms of their constituent particles and forces holding them together. 2. Construct and use atomic symbols and balanced equations to summarise chemical equations eg; neutralization, precipitation and combustion. 3. The sustainable management of a resource. 4. Chemical change in terms of energy. Priority 1 2.1: Ions – cations and anions, ionic formulae Molecules – covalent bonds, naming Solutions – solvent, solute, concentration, solubility curves 2.2 Chemical Reactions: Physical and chemical change – characteristics, types, exothermic and endothermic reactions. Chemical Equations – word and symbol, balancing equations 2.3 Reaction Types: combination, decomposition, precipitation, combustion, neutralisation and displacement. Solubility 2.4: Acids and Bases – properties, pH, chemical reactions, strong and weak acids, indicators. Revision Test Name______________________________________ Vocabulary mixtures compounds elements solutions solvent solute bonding: ionic, metallic, covalent physical and chemical change reactants and products concentration solubility Suggested Activities Ions Ionic formulae Exothermic Endothermic Chemical equations Acids Bases pH indicators dilute Assessment combustion neutralisation precipitation molecule atom electron anion/cation Practical write up – student choice from pracs listed in suggested activities Research report – student choice from research listed in extension & variation Test Extension and Variation Questions: 1-7 (p43), 18 – 24 (p44) Prac: Study of a reaction (p58) Activity: Ionic formulas sheet Homework Book: 2.1 Ionic compounds (p16-17) Prac: Colours of transition metal ions (p45) Prac: Solubility of potassium chlorate (sheet) Science at work: 2.1 p44 Questions: 10-13(p43) Investigate: Find out more about ionic, metallic and covalent bonding (p57) Questions: More balancing equations (sheet) Science at Work: Solubility curves (p44) worksheets. Questions: 1-10 (p52) Prac: Signs of chemical change (p 53) Homework Book: 2.2 Chem and Phys Change (p18-19) Prac: Endo and exo-thermic Reactions (sheet) Questions: 11-15, 19 - 25 (p52) &,25 (p59) extra sheets Homework: 2.3 Classifying reaction types page 2 (p21) Research: Researching bioluminescence (p53) Investigate: Types of chemical reactions Prac: Decomposition reactions, Precipitation of unknowns and Electroplating (p60-61) Questions: 1-16, 21&22 ( p59) Homework: 2.3 Classifying reaction types page 1 (p20) Investigate: The reactions and properties of acids and bases. Questions: 1-17(p.67) Prac: Common Indicators (p68), Natural Indicators (p69), Testing household solutions (p70), Acid reactions with metals and carbonates(p71) Research: What am I? Using solubility rules to identify unknown substances. Research: Look at how different animals stings work. Or Anaphylactic shock. Done @ School Done @ Home Teacher Signs Teacher signs