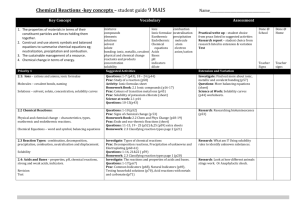
Lesson plan
advertisement
DISSOLUTION OF IONIC AND COVALENT SUBSTANCES Year 11 Chemistry – Aqeuous Solutions and Acidity Australian Curriculum learning objectives ACSCH0651: The solubility of substances in water, including ionic and molecular substances, can be explained by the intermolecular forces between species in the substances and water molecules, and is affected by changes in temperature. Resources required Water: A Unique Chemical captioned video from VEA. (Duration: 25 minutes.) Why do substances dissolve in water? Worksheet x class set Solubility Curve2 worksheet x class set Lesson outcome: students can explain how, using intermolecular forces, water is able to dissolve ionic and covalent compounds. Lesson outline 1. Students watch video with captions turned on. Students should watch the entire video to provide context, but the teacher should focus on the “Water: The nearly universal solvent” section. 2. Hand out the Why Do Substances Dissolve in Water? worksheet for students to read and complete. 3. Hand out the Solubility Curve worksheet for students to read and complete with reference to the solubility curve chart included. The relationship between solubility and temperature should be highlighted. Opportunity for further activity Students can do an investigation to determine the solubility of different ionic and covalent compounds by adding them to water and observing what happens. Students can do an investigation for one substance (e.g. sodium chloride) to determine the relationship between temperature and solubility and create their own solubility curve, by adding small amounts of solute to 100ml of water 1 http://bit.ly/1mOiq3M 2 http://bit.ly/1fQjJri of known temperature – increasing the temperature using a hot plate, adding more until it is saturated, and continuing.