Tissues in the lungs
advertisement

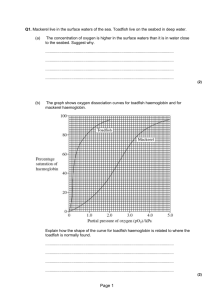
Hind Leys Biology F211 Transport in animals 5.6 Transport of oxygen Objectives Describe the role of haemoglobin in the transport of oxygen. Explain the significance of the different affinities for oxygen of foetal haemoglobin and adult haemoglobin. Haemoglobin Oxygen is transported in the red blood cells, or erythrocytes, which contain the protein haemoglobin. When oxygen combines with haemoglobin it becomes oxyhaemoglobin. Haemoglogin + Oxygen → Oxyhaemoglobin Hb + 4O2 → HbO8 Simplified to Hb + O2 → HbO2 Haemoglobin is a complex protein with four subunits. Each subunit consists of a polypeptide (protein) chain and a haem (non-protein) group. The haem group contains a single iron ion, Fe2+. This group has an affinity for oxygen, and each haem group can bind one oxygen molecule. In total, a haemoglobin molecule can carry four oxygen molecules. Figure 1 Haemoglobin structure 1. Describe the meaning of the term ‘affinity’. 2. Explain how red blood cells are adapted for the uptake and transport of oxygen. Just small enough to pass thru capillaries-short diffusion path, flattened shape increases surface area and increases exposure of Hb to O2, donut shape allows flexibility, no organelles so more space for Hb, filled with Hb- O2 not v water sol so only small amount transported in plasma 3. Red blood cells contain some enzymes which are involved in carbon dioxide transport. Explain why these enzymes cannot be replaced. 1 Hind Leys Biology F211 Transport in animals 5.6 Taking up and releasing oxygen Oxygen diffuses into the blood from the lungs. From the plasma, they diffuse into red blood cells and are bound to haemoglobin. This takes the oxygen molecules out of solution and so helps to maintain the steep concentration gradient, allowing more oxygen to enter the blood. In the tissues oxygen is needed for aerobic respiration. Oxyhaemoglobin must be able to release its oxygen where it is needed in the tissues. This is called dissociation. Haemoglobin and oxygen transport The ability of haemoglobin to take up and release oxygen depends on the amount of oxygen in the surrounding tissues. This is measured by the relative pressure that oxygen contributes to a mixture of gases, known as the partial pressure, pO2. It is also known as the oxygen tension and is measured in kilopascals, kPa. Haemoglobin can take up oxygen in a way that produces as S-shaped curve called an oxyhaemoglobin dissociation curve.It is S-shaped because of the behaviour of the Haemoglobin is different at different pO2. It shows that at the higher and lower partial pressures, there isn’t much change in the saturation of the Hb. But in the middle range, a small change in the pO2 can result in a large change in the percentage saturation of the blood. Venous pO2 Arterial pO2 Figure 2 Oxyhaemoglobin dissociation curve Explaining the shape of the curve At low pO2, haemoglobin does not readily take up oxygen. This is because the haem group is in the middle of the haemoglobin molecule. This makes it difficult for the oxygen molecules to diffuse in and associate with the haem group. This difficulty is responsible for the flat part of the curve at low oxygen tension. 2 Hind Leys Biology F211 Transport in animals 5.6 As the oxygen tension rises, the diffusion gradient into the haemoglobin molecule increases. An oxygen molecule eventually diffuses into the haemoglobin and associates with one of the haem groups. This causes a slight change in shape of the haemoglobin, a conformational change. This allows further oxygen molecules to diffuse in and bind more easily, accounting for the steep part of the curve. Once 3 O2 molecules are bound, it becomes more difficult for the final molecule to diffuse in and bind. It is therefore difficult to achieve 100% saturation of all haemoglobin molecules, even at very high pO2, shown by flat part of curve. Mammalian haemoglobin is well adapted to transporting oxygen to the tissues. The oxygen tension in the lungs is sufficient to produce almost 100% saturation. In the respiring tissues it is sufficiently low to cause oxygen to dissociate readily from the oxyhaemoglobin. 4. When the body is at rest, only one of the four oxygen molecules carried by haemoglobin is normally released into the tissues. Suggest why this could be an advantage when the organism becomes more active. 3 Hind Leys Biology F211 Transport in animals 5.6 The Bohr effect In the presence of carbon dioxide, haemoglobin changes shape allowing it to bind more loosely to oxygen. The amount of O2 carried and released by Hb depends on pH. Low pH (due to high CO2) causes HbO2 to dissociate from O2. A small decrease in the pH results in a large decrease in the % saturation of Hb with O2, even if pO2 is high. The more CO2, the more the curve shifts to the right Figure 3 The effect of pCO2 on oxyhaemoglobin dissociation . ANY SHIFT TO THE RIGHT = DECREASED AFFINITY FOR O2 The graph can be used to explain the following points: o There is high [O2] in the lungs and low [CO2] o The haemoglobin will therefore be virtually saturated with O 2. o In tissues (actively respiring) there will be low [O2] and high [CO2] The haemoglobin will therefore give up a lot of the O2 that it is transporting. 5. Explain the difference in the percentage saturation of haemoglobin at high pCO2. In summary, At the gas exchange surface carbon dioxide is constantly being removed. The pH is raised due to the low level of carbon dioxide. The higher pH changes the shape of haemoglobin into one that enables it to load oxygen rapidly. This shape also increases the affinity of haemoglobin for oxygen, so it is not released while being transported in the blood to the tissues. In the tissues, carbon dioxide is produced by respiring cells. Carbon dioxide is acidic in solution, so the pH of the blood within the tissue is decreased. 4 Hind Leys Biology F211 Transport in animals 5.6 The lower pH changes the shape of haemoglobin into one with a lower affinity for oxygen. Haemoglobin releases its oxygen into the respiring tissues. Foetal haemoglobin and myoglobin A foetus needs to be able to extract O2 from an environment which makes adult Hb release it. Foetal Hb has a higher affinity for O2 than adult Hb so picks up O2 from the blood, reducing pO2 and making adult Hb release it. Figure 4 Dissociation curves for foetal Hb and myoglobin The curve for foetal Hb lies to the left of the normal curve. ANY SHIFT TO THE LEFT = INCREASED AFFINITY FOR O2 6. The pO2 in the placenta may be 4kPa. What percentage of the mother’s haemoglobin will dissociate? 7. At this oxygen tension, what percentage of foetal haemoglobin will become saturated? 8. Explain the advantage of the foetal haemoglobin having a different curve to the mother’s. A foetus starts to make more adult haemoglobin towards the time of birth. After birth, the proportion of foetal haemoglobin in the blood declines quite rapidly. 9. Explain why it is important for the baby to have adult haemoglobin after birth. 5 Hind Leys Biology F211 Transport in animals 5.6 Myoglobin is a pigment found in muscles that is very similar to haemoglobin. Like haemoglobin it contains haem but each molecule contains only one polypeptide chain and one haem group. 10. How many oxygen molecules can a molecule of myoglobin combine with? 11. Use the graph to explain how the myoglobin in a muscle acts as an oxygen store that is used when the muscle is particularly active. 12. How can the oxygen store in myoglobin be replaced after use? During exercise, the body temperature rises due to the heat produced by increased respiration in the muscles. 13. Which way has the curve shifted and what is the significance of this? Shift to the right. Therefore increasing the release of O2 to respiring muscles during exercise Figure 5 The effect of temperature on oxyhaemoglobin dissociation 14. Complete the following table showing the affinity of haemoglobin for oxygen under different conditions. Region of body O2 CO2 Affinity of Hb Effects concentration concentration for oxygen Lungs High Low High Association Tissues Low High Low Dissociation This work can be reinforced using pages 79-86 of your textbook. 6