Elements and Bonding Worksheet
advertisement
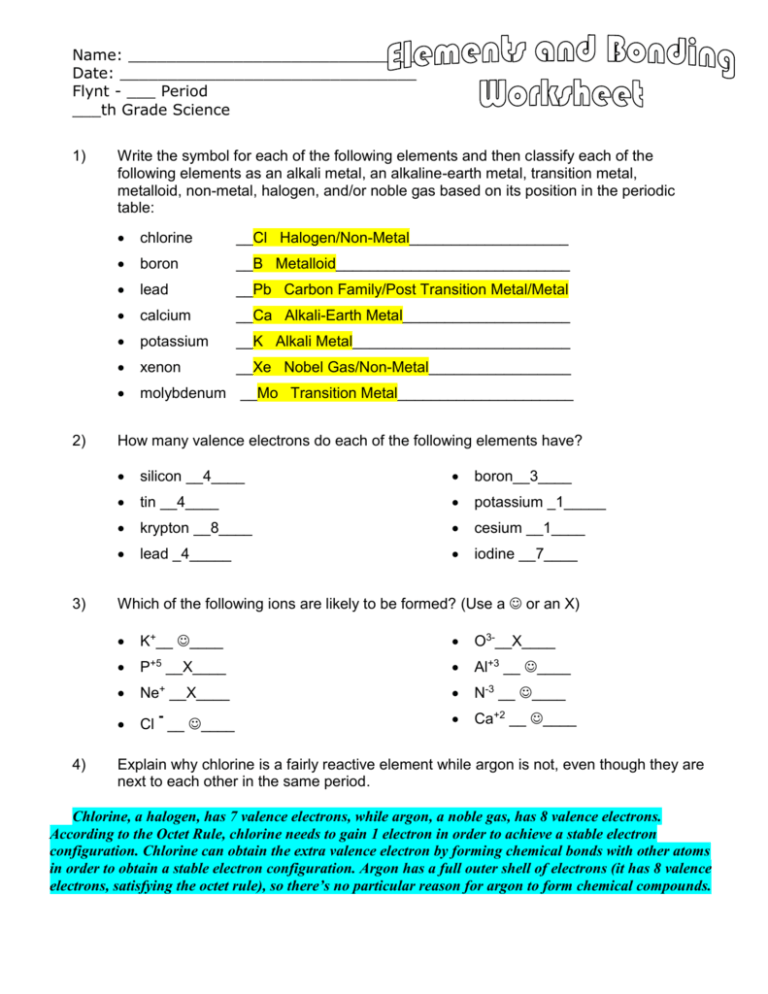
Name: ______________________________ Date: _______________________________ Flynt - ___ Period ___th Grade Science 1) 2) 3) 4) Write the symbol for each of the following elements and then classify each of the following elements as an alkali metal, an alkaline-earth metal, transition metal, metalloid, non-metal, halogen, and/or noble gas based on its position in the periodic table: chlorine __Cl Halogen/Non-Metal___________________ boron __B Metalloid____________________________ lead __Pb Carbon Family/Post Transition Metal/Metal calcium __Ca Alkali-Earth Metal____________________ potassium __K Alkali Metal__________________________ xenon __Xe Nobel Gas/Non-Metal_________________ molybdenum __Mo Transition Metal_____________________ How many valence electrons do each of the following elements have? silicon __4____ boron__3____ tin __4____ potassium _1_____ krypton __8____ cesium __1____ lead _4_____ iodine __7____ Which of the following ions are likely to be formed? (Use a or an X) K+__ ____ O3-__X____ P+5 __X____ Al+3 __ ____ Ne+ __X____ N-3 __ ____ Cl __ ____ Ca+2 __ ____ Explain why chlorine is a fairly reactive element while argon is not, even though they are next to each other in the same period. Chlorine, a halogen, has 7 valence electrons, while argon, a noble gas, has 8 valence electrons. According to the Octet Rule, chlorine needs to gain 1 electron in order to achieve a stable electron configuration. Chlorine can obtain the extra valence electron by forming chemical bonds with other atoms in order to obtain a stable electron configuration. Argon has a full outer shell of electrons (it has 8 valence electrons, satisfying the octet rule), so there’s no particular reason for argon to form chemical compounds. 5) Explain why fluorine and chlorine have similar reactivities. (The word “valence” should be somewhere in your answer!) As members of the Halogen Group, both fluorine and chlorine have the same number of valence electrons (seven). Since valence electrons determine how an element will react chemically with other elements (chemical reactivity), having the same number of valence electrons gives fluorine and chlorine similar reactivities. Both elements need one additional electron to meet the Octet Rule and achieve a stable electron configuration. Therefore both chlorine and fluorine tend to bond with elements that readily provide the needed extra electron (elements with low electron affinities and low ionization energies). 6) Explain why magnesium tends to lose electrons when becoming an ion, while sulfur tends to gain electrons. With two valence electrons, it’s easier for magnesium to lose its two valence electrons rather than gain six additional electrons in order to meet the octet rule and achieve a stable electron configuration (thus becoming like the nearest noble gas). This is why magnesium has an oxidation number of 2+, indicating that it tends to become a cation with a 2+ charge. In contrast, sulfur—with its six valence electrons—can most easily meet the octet rule by gaining 2 additional electrons rather than by losing its 6 valence electrons. Thus, sulfur’s oxidation number is 2-, indicating it typically becomes an anion with a 2- charge. 7) Research and describe at least 5 unique traits/characteristics of ions, ionic bonds, and ionic compounds. From Hyperphysics.com: An ionic bond forms when one or more electrons from one atom are removed and attached to another atom, resulting in positive and negative ions which attract each other. In chemical bonds, atoms can either transfer or share their valence electrons. In the extreme case where one or more atoms lose electrons and other atoms gain them in order to produce a noble gas electron configuration, the bond is called an ionic bond. Ionic bonds often form between the alkalis and the halides, such as sodium chloride, NaCl. From VisionLearning.com: In ionic bonding, electrons are completely transferred from one atom to another. In the process of either losing or gaining negatively charged electrons, the reacting atoms form ions. The oppositely charged ions are then attracted to each other by electrostatic forces, which are the basis of the ionic bond. For example, during the reaction of sodium with chlorine (in the picture at left), sodium (grey atomic model) loses its one valence electron to chlorine (green atomic model). In the picture below right, you can see that this electron exchange results in a positively charged sodium ion and a negatively charged chlorine ion. Notice that when sodium loses its one valence electron it gets smaller in size, while chlorine grows larger when it gains an additional valence electron. This is typical of the relative sizes of ions to atoms. Positive ions tend to be smaller than their parent atoms while negative ions tend to be larger than their parent. After the reaction takes place, the charged Na+ and Cl- ions are held together by electrostatic forces, thus forming an ionic bond. Ionic compounds share many features in common: Ionic bonds form between metals and nonmetals. In naming simple ionic compounds, the metal is always first, the nonmetal second (e.g., sodium chloride). Ionic compounds dissolve easily in water and other polar solvents. In solution, ionic compounds easily conduct electricity. Ionic compounds tend to form crystalline solids with high melting temperatures. From Wikipedia: An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some degree of covalent bonding. Thus, an ionic bond is considered a bond where the ionic character is greater than the covalent character. The larger the difference in electronegativity between the two atoms involved in the bond, the more ionic (polar) the bond is. Bonds with partially ionic and partially covalent character are called polar covalent bonds. Ionic bonding is a form of non-covalent bonding. Ionic compounds conduct electricity when molten or in solution, but not as a solid. They generally have a high melting point and tend to be soluble in water. 8) Pick two elements that might be likely to form an ionic bond. Use the number of valence electrons to explain why you think these two elements might form an ionic bond. Answers may vary. Ionic bonds occur between metals and non-metals on the periodic table. 1) For example, consider Na and Cl. Sodium would lose its lone valence electron and become positively charged and the chlorine would gain one electron to fill its valence shell, becoming negatively charged. The positive/negative charge attraction would hold the two ions together. 2) Another example, magnesium and oxygen. The magnesium would lose its two valence electrons, becoming +2 charged and the oxygen would gain the two electrons to fill its valence shell, becoming -2 charged in the process. The positive/negative charge attraction (this time +2/-2, four times as much as the +1/-1 of NaCl) would hold the two ions together. 3) Last example, Mg and Cl. The magnesium would lose two electrons, becoming +2 charged and two chlorines (not one) would each gain one electron, each becoming -1 charged in the process. The three ions would adhere (bond) to each other by the positive/negative attraction between the ions.






