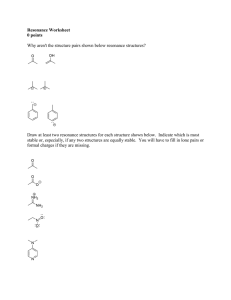
1. Opposites attract, likes repel. It takes energy to separate two
advertisement

1. Opposites attract, likes repel. It takes energy to separate two unlike charges or to make like charges come together. This is true in a molecule or in a solution. 2. Lower energy system are more stable. Higher energy systems tend to react more quickly or do not not need a catalyst. Lower energy systems require input of energy to make them react. 3. Resonance stabilizes. The more resonance structures you can draw, the more stable a system will be. The system will average out to resemble the more stable resonance structures you draw. Charges (that might cause a system to be unstable) are stabilized by spreading a charge through a molecule by resonance structures. 4. Carbons like to have four bonds and like to be neutral in charge. Carbonium ions are stable in the order tertiary>secondary>primary, and are stabilized by groups that introduce electron density (such as additional carbon groups). Carbanions are stable in the order primary>secondary>tertiary and are stabilized by groups that drawing electron density (such as more electronegative groups). Double bonds are stabilized by more carbon and carbon-containing groups attached to them. 5. Oxygen atoms draw electrons to themselves. Carbonyls (>C=O) distribute electron density where the oxygen draws more electrons to itself (it gets a partial negative charge and the carbon gets a partial positive charge). The same is true with alcohols, etc. Nitrogens are not as electronegative as oxygens and have less, although not insignificant, effect. Hydrogens (not acid groups) are similar to carbons but less electron donating. 6. Carbons alpha to a carbonyl are relatively acidic. The resonance structure stabilizes the system, as electrons are pulled onto the oxygen. This is also important to consider for any system that contains a carbonyl, such as a carboxylic acid. It is not unusual to have a carbonyl or carboxylic acid in an alpha/beta unsaturation, as this is relatively well stabilized by charge distribution and resonance. 7. Charges are conserved! Always be sure to balance or account for charges formed or eliminated. The NET charge in a reaction system (including solvent) does not change. To remember this, imagine you will generate massive thunderbolts from your test tube reaction as nature will try to conserve charges. 8. Like dissolves like. Oil and water do not mix. Solvents closer in character to oil (less polar, more aliphatic) will better dissolve oil. Solvents closer to the character of water (more polar, less aliphatic) will mix with water. 9. The stronger the acid, the weaker the conjugate base and the conjugate base tends to be a better leaving group. Think of this when a group might be hydrolyzed or otherwise reacted off a molecule. Imagine an acid like hydrochloric acid compared to stearic acid. In water, the HCl is much more readily reacted into its two components (H+ and Cl-). Stearic acid (a long-chain fatty acid) tends not to be as readily ionized. So, generally, halides would be better leaving groups than an organic acid. 10. Chemicals and chemical reactions seek the lowest energy state. A reaction will proceed only if the products are more stable than the the reactants. A high energy threshold must be overcome (a suitable catalyst can lower a high energy threshold) before a reaction can proceed.