Introduction to BIO 151 (Week 1)
advertisement

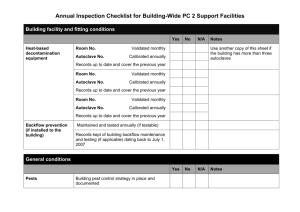
Version: 2/18/2016 Introduction to BIO 151 (Week 1) This handout contains basic information and skills that you will need to successfully design, execute, and complete an independent research project in forensic biology this semester. Because this is an advanced inquiry-based course, students will be responsible for all aspects of their research projects, including experimental design, preparing their own materials and reagents, choosing the appropriate lab water and mixing tools, disposing of biohazardous and chemical waste, and cleaning up at the end of each lab day. We will discuss the material in this handout on the first day of class and you will be given a homework assignment to help you practice and hone your skills and understanding. It is vital that you pay close attention to the information below so that you can work safely, efficiently, and effectively in the lab. I. Safety and Cost Considerations Gloves. Gloves should be worn at all times. To save money, please change your gloves sparingly and think critically about whether or not changing gloves is actually necessary. Unless you have an allergy to latex, please use the latex gloves. Nitrile gloves are more expensive. Bench Diapers. Always work on a piece of bench diaper. Change the diaper if it gets wet or dirty or if keeping the same piece of bench paper in place may lead to cross-contamination of evidence. Think critically about whether or not it is necessary to change your bench diaper. Cleaning Scissors and Tweezers. As in BIO 150, you will be using three solutions to clean your scissors and tweezers between uses: 10% bleach (to destroy DNA), dI water (to remove the bleach), and ethanol (to destroy any bacteria or other microorganisms). Bleach is fairly effective at preventing cross-contamination of DNA from one evidence item to another, but is not full-proof. You may wish to clean your non-disposable instruments more than once between uses to make sure to avoid inadvertently transferring minute amounts of DNA from one item to another. In addition, you should always perform your negative controls first, your experimental samples second, and your positive controls last. You can save your bleach and water beakers by covering them with seran wrap and placing them in your lab cabinet between labs. However, the beakers containing ethanol should be emptied into the ethanol container in the chemical hood for storage in a flame proof cabinet between uses. Autoclaving. Think carefully about whether or not items need to be autoclaved. Sometimes it is not necessary to sterilize instruments and reagents, while other times it is very important. Look at the labels on reagents and items. Do the items come pre-sterilized (e.g. filter tips, disposable FOR 151 Fall 2010 Page 1 Version: 2/18/2016 scalpels)? If so, sterilizing them is a waste of time and resources. In addition, although you need to clean your non-disposable implements (scissors, tweezers), you do not need to sterilize them between uses (except by briefly cleaning them with ethanol). Likewise, you can clean beakers and other types of glassware with soap and tap water; in most instances, you do not need to sterilize these items prior to use. Your decision on whether or not to autoclave items will depend on whether contamination with bacteria is likely to interfere with your downstream analyses, whether the item you are examining is already compromised with bacteria, and whether the reagent is subject to bacterial contamination. For example, you do not need to autoclave ethanol since bacteria cannot grow in it! Try to think critically and logically about sterilization, and never be reluctant to ask questions if you are confused. Remember that sterilizing items is time-consuming and expensive. More information on autoclaving is provided in Section IV of this handout. Lab water. Review Section V of this handout as a guide to choosing the right type of lab water for the task at hand. Know the differences between tap, deionized, distilled, and nuclease-free waters and when it is important for water to be sterile. Highly purified waters are critical for some applications but are very expensive are not necessary for most applications. For example, rinsing glassware in nuclease-free water would quickly bankrupt a lab, but failing to use nuclease water when setting up a PCR reaction could result in DNA degradation and the loss of probative genetic information from a sample. Again, don’t hesitate to ask questions if you are confused and need some guidance on which type of water to use. Biohazardous and Chemical Waste. Biohazardous waste is any organism, or its toxins, that can cause disease in animals or humans. Biohazardous waste is produced by living organisms and can cause damage to other biological organisms. Most biohazardous waste can be neutralized with bleach or by autoclaving. In contrast, Chemical waste is waste made from harmful chemicals. It is non-living and usually cannot be neutralized by bleach or autoclaving (either of which might actually do more harm than good). Chemical waste should therefore always be collected in separate collection vessels from biohazardous waste. In addition, each type of chemical waste is subject to different neutralization procedures and so must be collected separately from other types of chemical waste. If an item has both biohazardous and chemical waste properties, the neutralization of its biohazardous component takes precedence. However, since the item also contains hazardous chemicals, the method of rendering it free of biohazardous materials must be chosen very carefully. In lab, we will have several, clearly labeled containers for collection waste. Be sure to use the right collection vessel and ask if you need help. Material Safety Data Sheets (MSDS) are available for almost every chemical, and companies that make reagents with proprietary formulae must disclose the chemical and/or biohazardous properties of their products. MSDS information can be used to determine whether a chemical or FOR 151 Fall 2010 Page 2 Version: 2/18/2016 solution is biohazardous or contains chemical waste and how to dispose of it appropriately. Fortunately, in most laboratories (including this one) waste disposal is delegated to specialized personnel, who are an excellent source of information and help to researchers and technicians. These specialists supply labs with labels, collection vessels, and waste protocols that meet government guidelines, and run central facilities where labeled waste can be deposited. MSDS access will be provided; however, you will be given clear directions on where to dispose of materials, so you probably will not need to access the MSDS materials while you are taking this course. II. Calculations and Measurements Performing accurate calculations and measurements is absolutely critical in a molecular biology laboratory. To succeed, you will need to be fluent in unit conversions, know which equations to apply for which types of calculations, know the capabilities and limitations of your equipment, and be able to maintain your equipment for optimal performance. For example, micropipetters have set ranges and should not be used outside of those ranges. In addition, they should be calibrated regularly (about once every 6-12 months is optimal). Homework 1 is designed to help you test your current skills and, if they are deficient, to become comfortable solving routine types of lab calculations. Remember: you are striving for fluency, which means that you should be able to set up and solve problems easily and automatically. You should also be able to quickly identify what additional information (if any) you will need to solve the problem and how to get it. 1. Measuring Volumes of 1 uL - 1 mL: Use and Maintenance of Micropipetters Micropipetters are indispensable to molecular biologists because they allow the accurate measurement of volumes between 1uL and 1 mL - the typical volume range used for most molecular biology lab work. If you have not done so already, it is very important that you commit to memory the following simple units of the metric system of measurement. You will use them often! “milli” (m)= 10-3 “micro”) (µ) = 10-6 “nano” (n) = 10-9 “pico” (p) = 10-12 For example, 650 mL = 0.65 L and 200 uL = 0.2 mL. If these types of conversions are not second nature (and very easy to do in your head), start practicing! FOR 151 Fall 2010 Page 3 Version: 2/18/2016 The micropipetters we will use are called Finnpipettes (Fisher Scientific). Each instrument is calibrated for a narrow range (0.5-10 µL, 5-50 µL, 20-200 uL, and 100-1000 µL) and is a different color. The 0.5-10 uL micropipetter is used with microtips when barrier filters are needed (e.g. for DNA work) but can be used with minitips for most other purposes. The 5-50 uL and 20-200 uL pipettes are used exclusively with minitips and the 100-1000 uL pipette is used with macrotips. Fortunately, if you try to put the wrong size tip on a micropipetter, it will not fit. Unfortunately, it is easy to confuse 20 uL with 200 uL (etc.) if you are not familiar with the instruments. Everyday use will limit such mistakes; however, it is important to be very careful! Fill in the far right column of the table below. If you have questions, ask! READING THE VOLUME WINDOWS ON THE FINNPIPETTES READING IN VOLUME SAMPLE VOLUME OF SAMPLE READING WINDOW AT TOP VALUE READING (FILL IN) 200-1000 uL 1-0-0 0-7-7 20-200 50-200 uL 2-0-0 0-7-7 5-50 5-50 uL 5-0-0 0-7-7 0.5-10 0.5-10 uL 5-0-0 0-7-7 PIPETTER RANGE 100-1000 To suck liquid into the tip: Gently press down on the plunger to the first stop position (the position where you will first feel resistance to your pressing.) Then place the tip into the liquid to be measured (below the level of the liquid’s surface) and gently and slowly release the pressure on the plunger. Then remove the pipette from the liquid and transfer the contents in the tip to the container you wish to fill. (The liquid will remain in the tip as you do this because it is being held in by the vacuum you created inside the micropipetter when you released the pressure on the plunger.) To expel the liquid from the tip: Gently push the plunger down to the first stop position and then continue until you reach the second (final) stop position. You may use either your thumb or your first finger to depress the plunger, whichever is most comfortable. If you are transferring the contents of the tip into a tube that already contains liquid, gently express the contents into the liquid, below the level of its surface. If you are transferring the contents of the tip into an empty tube, place the tip on one of the inside surfaces of the tube while expelling. (This will allow capillary action to help you release all the contents of the tip into the tube; if you do not do this, some liquid may remain in the FOR 151 Fall 2010 Page 4 Version: 2/18/2016 tip.) As you expel, keep the pressure on the plunger. Do not release the pressure until you have removed the tip from the tube. (Otherwise you’ll just suck the liquid right back up into the tip!) The best technique is to watch the liquid as you are micropipetting to be absolutely sure that you get the correct amount in the pipette tip at the start of the process (no bubbles) and that all of it was expelled at the end. Experienced lab technicians develop a good sense of how different volumes appear in a tip. This helps them avoid pipetting errors, particularly those that occur when they accidentally pick up the wrong pipetter. The micropipetters we will be using have an ejector on one side. After use, the tip can be popped off by pushing down on the ejector button at the top of the pipetter. This avoids the necessity of having to remove the pipette tip with your fingers. 2. Weighing Reagents To accurately measure the weight of a dry substance, particularly when the amount is small, plastic weigh boats or weigh papers are a good choice. A small spatula is used to carefully transfer the powdered reagent to the weigh boat or paper and the substance is then weighed on a scale. Since the weight of the boat or paper needs to be subtracted from the total weight in order to get an accurate measurement, the weigh boat or paper is first placed on the scale and the scale is "zeroed" or "tared" (set to 0) before the substance is added for weighing. Scales have different levels of sensitivity and it is important to know the limits of the scale you are using. This is usually displayed on the back or side of the scale. When making solutions in the lab, it is critical that you know the difference between weight, moles, molecular weight, and molarity. These terms are defined below. Make sure you commit them to memory if you don’t already know them. Weight is a measurement of the gravitational force acting on an object. Near the surface of the Earth, the acceleration due to gravity is approximately constant; this means that an object's weight is roughly proportional to its mass wherever the measurement is made (e.g. Sacramento or Zanzibar). Therefore, because all our measurements will be made on planet Earth (there will be no field trips to Venus or Mars this quarter!) we will use the terms weight and mass interchangeably. Please remember, however, that weight and mass are not equivalent terms in physics. A mole is defined as 6.022 ×1023 atoms or molecules of a substance. This may seem like a ridiculously high number, but given the small size of most molecules used in molecular biology, it is actually very useful. FOR 151 Fall 2010 Page 5 Version: 2/18/2016 Molecular weight (MW) or formula weight (FW) is defined as the weight of one mole of an atom or molecule. The most common unit of expression for molecular weight is g/mol. NaCl is a very small molecule, so 6.022 ×1023 molecules (one mole) of NaCl doesn’t weigh much (58.44 grams or about 2 ounces). On the other hand, one mole of human chromosome 1 (a molecule containing 247 million base-pairs of DNA, with a MW of 660 g/mol per DNA base-pair) weighs a lot more. Use the space below to calculate the MW of chromosome 1. Assuming that there are 6 billion human beings on Earth and each human body contains about 10 trillion cells, how many moles of human chromosome 1 are on the planet? Taken together, how much do all chromosome 1’s on the planet weigh? Calculate the number in grams, pounds, and tons. (Note: There are 453.6 g per pound and 2,000 pounds per ton.) To give you some perspective, the U.S.S. Missouri (a typical battleship) weighs about 45,000 tons. Molarity (M) is defined as the number of moles per liter of a solution of a substance. Obviously, the molarity of a solution can be manipulated by a researcher since it depends on how much of the substance is added to how much solvent (usually water). For example, to make 1 liter of a 1 M solution of NaCl would require 58.44 g of NaCl. To make 1 L of a 0.1 M solution, you would need only 5.844 g. (How many grams of chromosome 1 would you need to make up 1 L of a 1M solution?) 3. Working with Solutions The concentrations of solutions in the lab are usually given in molarity (M = moles/L). However, they may also be expressed in grams/L, as a percent, or as an “X” (e.g. 10X, 1X, etc.). By definition, 1 g/mL = 100%, so conversions can easily be made between the first three methods of expressing solution concentration (assuming that you know the FW of the substance). “1X” is usually the working concentration of a solution. Without additional information, you can’t convert “X” to M, g/L, or a percent. However, if your stock solution is at 10X, you can easily make a 1X solution by diluting the solution 10-fold. FOR 151 Fall 2010 Page 6 Version: 2/18/2016 Here’s a practice problem: NaCl has a FW of 58.44. You have a 5 M solution of NaCl. What is its concentration in g/L? What percent solution is it? (5 moles/L)(58.44 g/mole) = 292.2 g/L (292.2 g/L)(100%/(1 g/mL)(1 L/1000 mL) = 29.2% You will need to learn to do these kinds of conversions quickly and easily. Make a habit of always including units and cancelling them out (no shortcuts!). You will catch errors much more easily if you do this. It also helps to identify first what units you want for your final answer. This unit should be in the numerator in one of the fractions in your conversion. For example: How many moles are in 3.0 mL of a 10.0 g/mL solution of NaCl? You want moles in your answer, so moles should be in the numerator of one of the fractions in your calculation. (1 mole/58.44 g)(10 g/mL)(3 mL) = 0.51 moles In addition, you will find the following formula very helpful when working with solutions in the lab: (Vi)(Ci) = (Vf)(Cf) Where: Vi = initial volume Ci = initial concentration Vf = final volume Cf= final concentration Suppose, for example, that you have a stock solution of EDTA at 2 M. You wish to make 50 mL of a 0.8 M solution of this reagent. How would you do it? Vi = (Vf)(Cf) = (50 mL)(0.8 M) Ci = 20 mL 2M Assuming that the solvent is dI water (in molecular biology it usually is), you would add 20 mL of 2M EDTA to 30 mL of dI water to make 50 mL of 0.8 M EDTA solution. III. Lab Water For the purposes of this class, we will divide our water supply into the two types usually available in molecular biology laboratories: tap and purified. Each can then be sub-divided into classes, as shown in the table. FOR 151 Fall 2010 Page 7 Version: 2/18/2016 Type Method of Purification Properties Tap Industrial None Contains ions, organic compounds (natural and manmade), particulates (vegetable debris, sand, and colloids), microorganisms (>1 cfu/mL), DNAases, and RNAases; Free of microorgansims and DNAases if sterilized Drinking Gross filtration (sand, activated charcoal) and disinfection (chlorine, UV) Contains ions, organic compounds (natural and manmade), microorganisms (< 1 cfu/mL), DNAases and RNAases; Free of microorganisms and DNAases if sterilized Ion exchange resin or electrodeionization Very low levels of mineral ions; contains organic compounds, microorganisms (<1 cfu/mL), DNAases and RNAases; Free of microorganisms and DNAases if sterilized Distilled Distillation Very low levels of ions and organic molecules; microorganisms may also be present at low levels, DNAases and RNAases. Free of microorganisms and DNAases if sterilized Ultrapure Ultrafltration; reverse osmosis Free of ions, organic molecules and microorganisms but not necessarily DNAases and RNAases; DNAases can be removed by sterilizing Nuclease-free Ultrafiltration; reverse osmosis; DEPC treatment Free of ions, organic molecules, bacteria, DNAases, and RNAases. Purified dI (deionized) Industrial Water can be used for washing glassware, plastic ware, hands, etc., but it is not safe to drink. It usually comes directly from a tap located in a lab sink and is often labeled as “Industrial water” or as “Unsafe to drink.” Once glassware or plastic ware has been cleaned with industrial water, it should always be rinsed thoroughly with dI water to remove any residual contaminants. Deionized (dI) water has largely replaced distilled water as a source of ion-free water. It is less expensive to produce than distilled water and, since most of the chemical contaminants in water are ions, removes the chemicals of most concern. dI water can be sterilized by autoclaving and is suitable for the preparation of most molecular biology lab solutions and for rinsing glassware and plastic ware after it has been washed in industrial water. Because it is does not contain minerals that leave deposits, it is also the best choice for filling water baths and piping into autoclaves and lab dishwashers. It is also used to prepare microbiological media prior to autoclaving (although sometimes it is better to use tap water because some microbes require ions for robust growth). Ultrapure water is made by filtering dI or distilled water (in a reverse osmosis system) through a FOR 151 Fall 2010 Page 8 Version: 2/18/2016 very fine filter (a pore size of 0.22 um is typical) to remove any residual ions and bacteria. However, DNAases or RNAases may still be present. DNAases can be removed by autoclaving since they are permanently denatured by exposure to heat. Autoclaved ultrapure water is the best choice for the preparation of any solution that will come into contact with DNA, including solutions for DNA extraction, quantitation, and PCR. However, it is not sufficiently pure for experimental solutions that require intact RNA (such as reverse transcriptase PCR). Nuclease-free water is ultrapure water that has been treated with DEPC (a non-specific inhibitor of RNAases) and then autoclaved (to destroy the DEPC and inactivate DNAases). RNAases are not destroyed by autoclaving because they tend to refold after being heat-denatured. Nuclease-free water is necessary for any lab work that requires intact RNA. IV. Autoclaving An autoclave is an essential piece of equipment in a molecular biology lab. It is used to sterilize solutions, supplies, small instruments, and biohazardous waste using high pressure steam (at 120 deg C or above). Steam is used because it can achieve sterilization much more quickly than hot air (15 minutes as opposed to several hours). Not all materials can survive the high temperatures and pressures produced in an autoclave cycle and it is therefore extremely important to use the right type of glassware and plastics. Thick Pyrex glassware and either PFA, polypropylene, polysulfone and Noryl plastics are usually autoclave-safe, but you should always check to make sure that the items you place in the autoclave can survive the procedure. Thin glass will break, and many types of plastics will melt, leaving a big mess and producing volatile products that may be toxic. Extreme care should be used when loading and unloading an autoclave. It is very easy to get burned – either by inadvertently touching the side of the autoclave with your uncovered arm or by failing to move out of the path of the steam as it escapes from the autoclave door. Long protective autoclave gloves should be worn at all times, and the door should be opened in such a way that your body is out of the path of the steam. It is always best to place items in an autoclave-proof tray during the run so that if breakage occurs it is contained. Trays also make removing items from the autoclave safer and easier. Autoclave tape changes color from white to striped brown during autoclaving. Placing autoclave tape on an item prior to autoclaving provides you a visual cue that the item or solution has been sterilized. FOR 151 Fall 2010 Page 9 Version: 2/18/2016 During autoclaving, some water will evaporate from your solutions. Therefore, to maintain the right concentration, you should use a Sharpie to mark the level of the fluid before autoclaving. After autoclaving, you should then add STERILE water to the solution to replace the water that was lost during the procedure. If you do not do this, your solution may be more concentrated than you intended, since only the water evaporates during the process (not the ions or other solutes). V. Mixing During molecular biology experiments, you will often need to mix two or more liquid ingredients together. Mixing is extremely important, and experiments can fail if you do not mix ingredients together thoroughly. However, it is important to know the various methods of mixing and when to apply them. Vortexing is fine under many circumstances but can be destructive in others. Usually, the protocol you are following will indicate what method of mixing is appropriate. If not, follow the general rule that solutions containing DNA, RNA, and proteins that are needed for downstream applications should be mixed using the flicking or pipette method. Most other mixtures are amenable to vortexing unless they contain detergents (e.g. lysis buffers) that will become bubbly when vortexed. 1. Vortexing Most molecular biology vortexers have 3 settings: Off, Continuous, and Touch. The Touch setting is preferred because the vortexer will not operate (and make a lot of noise) unless the researcher places the tube in the rubber holder and applies downward pressure. In the Continuous mode, the vortexer is on all the time, regardless of whether anything is being vortexed. Some vortexers have tube attachments that allow researchers to shake contents vigorously over an extended period of time. 2. Flicking, Pipetting, and Inverting The materials in tubes can also be mixed by gently flicking the tube several times with your finger. Alternatively, the contents can be gently mixed by pipetting the mixture up and down a few times. It is usually important not to introduce bubbles, so it must be done carefully and gently to be effective. Another method is to simply invert the tube several times, making sure the lid is firmly in place. VI. Supplies Boxes Each student will be assigned his/her own supplies box for use during the semester. The materials in your box are expensive and the box must always be locked in your lab cabinet between labs. Each box contains the following items, and you are responsible for making sure that nothing gets lost! FOR 151 Fall 2010 Page 10 Version: 2/18/2016 6” plastic ruler Set of four micropipetters (Finnpipettes) and rack One microcentrifuge tube opener Reversible rack for 1.5-mL/0.5 mL microcentrifuge tubes Rack for 0.2-mL PCR tubes Roll of lab tape (colored) Roll of autoclave tape Roll of evidence sealing tape Black ultrafine Sharpie marker Peel-off China marker Pair of tweezers Pair of scissors Lab timer 1 box small Whatman filter paper circles 1 dropper sterile, distilled water 1 pack of lens paper 1 magnifying glass During the first day of lab, you will be asked to inventory your box to make sure everything is present. You will then sign the inventory form and submit it to your instructor. VII. Tips, Tubes, and other Items Before beginning your lab work, you and your lab partner will need to gather together the following items, which you can store in your lab cabinet/drawer: One box Kim Wipes One 100-mL graduated cylinder One freezer box Two plastic collection beakers labeled “dry waste” and “liquid waste 2 full pipette tip racks (1 macro, 1 mini)* One large beaker filled with 1.5-mL tubes* One medium beaker filled with 0.5 mL tubes* Three 250-mL autoclave-ready bottles One 500-mL autoclave-ready bottle half-filled with dI water* Use the colored tape in your supplies box to label the items that are not in your supplies box with the bench numbers of you and your lab partner (e.g. 1/8). Use the Sharpie in your supplies box to label the tape; DO NOT label directly on the items with the Sharpie. FOR 151 Fall 2010 Page 11 Version: 2/18/2016 The items marked with an asterisk will need to be sterilized immediately. Follow the instructions below to autoclave these items: Place a piece of autoclave tape on the side and top of your tips boxes. Put a piece of lab tape on the side of each box and label it with your lab station number. Place the tip boxes on the autoclave cart.* Fill your large glass beaker with 1.5-mL microcentrifuge tubes and your medium glass beaker with 0.5 mL microcentrifuge tubes. Cover each with aluminum foil and adhere a small piece of autoclave tape to the top of the foil as well as on the side of the beaker. Label the beakers with your lab bench numbers (using your colored lab tape) and place the beakers on the autoclave cart when you are done. Add approximately 400 mL dI water to your 500-mL autoclave bottle. (Your instructor or T.A. will show you where the dI water taps are located.) Label the bottle with the name of the solution (“Sterile dI water”), your lab bench numbers, and today’s date. Place autoclave tape on the cap and side of the bottle and then place the bottle on the autoclave cart. Be sure to loosen the lid to prevent the bottle from breaking during autoclaving. Prepare your solutions of Tris and EDTA (see below). When all the items are ready, your instructor or T.A. will instruct you in the proper use of the autoclave. When your items are finished autoclaving, they will be removed from the autoclave and allowed to cool until the next lab period. When they are completely cool, you can store them in your lab cabinet. *The tips in these boxes can be used for most applications. However, you will be provided with pre-sterilized, nuclease-free, aerosol-barrier tips for your qPCR work. VIII. Prepare T10E0.1 Buffer You will eventually need TE buffer for qPCR. To practice making up a solution, you will prepare your TE buffer during the first week of lab, following the steps below. 1. Prepare 100 mLs of 1 M Tris-HCl, pH 8.0. The poly bottle of Tris Base is on the front bench. Using the FW on the bottle, calculate how much powdered Tris Base you will need. Weigh out the appropriate amount of Tris Base and place it in a 250-mL beaker Add 70 mLs dI water and 4.2 mL concentrated HCl. Add a stir bar, place the beaker on a stir plate, and stir until dissolved. FOR 151 Fall 2010 Page 12 Version: 2/18/2016 When the solution is clear, test the pH. Make minor adjustments to bring the pH to 8.0 using either concentrated HCl or NaOH crystals. Pour the solution into a 100 mL graduated cylinder and top off to exactly 100 mLs with dI water. Pour the solution into a 250-mL autoclave-ready bottle and mark the miniscus with your Sharpie. Label the solution “1 M Tris-HCl, pH 8.0” followed by today’s date and your lab bench numbers. Place a small piece of autoclave tape on the lid and side of the bottle, crack the lid, and place the bottle on the autoclave cart. 2. Prepare 100 mLs of 500 mM EDTA, pH 8.0 The poly bottle of EDTA is on the front bench. Using the FW on the bottle, calculate how much powdered EDTA you will need. Weigh out the appropriate amount of EDTA and place it in a 250-mL beaker. Add 80 mL dI water Add 2g NaOH pellets Add a stir bar, place the beaker on a stir plate, and stir until dissolved. (This may take a little while.) After the solution is clear, test the pH. Make minor adjustments to bring the pH to 8.0 using either concentrated HCl or NaOH crystals. Pour the solution into a 100 mL graduated cylinder and top off to exactly 100 mLs with dI water. Pour the solution into a 250-mL autoclave-ready bottle and mark the miniscus with your Sharpie. Label the solution “0.5 M EDTA, pH 8.0” followed by today’s date and your lab bench numbers. Place a small piece of autoclave tape on the lid and side of the bottle, crack the lid, and place the bottle on the autoclave cart. 3. After your autoclaved solutions have completely cooled, prepare a 5 mM solution of EDTA as follows: Use a micropipetter to add 9.9 mL sterile water to a 15-mL sterile Falcon tube. Add 0.1 mL of your 0.5 M EDTA solution. Invert to mix. Label the tube “5 mM EDTA, pH 8.0” followed by today’s date and your lab bench numbers. Calculate how much 1 M Tris (8.0) and 5 mM EDTA (8.0) you will need to make 10 mL of a solution that contains a final concentration of 10 mM Tris and 0.1 mM EDTA. Write out the recipe and check with your instructor or T.A. before proceding: FOR 151 Fall 2010 Page 13 Version: 2/18/2016 T10E0.1 Buffer Recipe (10 mL): ___________ mL 1M Tris (8.0) ___________ mL 0.05 M EDTA (8.0) ___________ mL sterile water Use micropipetters to mix together the ingredients in a sterile 15-mL Falcon tube. Label the tube “T10E0.1 Buffer” followed the date and your lab bench numbers. All your solutions should be stored in your lab cabinet for future use. FOR 151 Fall 2010 Page 14