Local Risk Assessment
advertisement

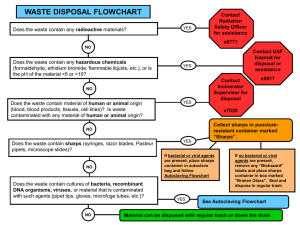
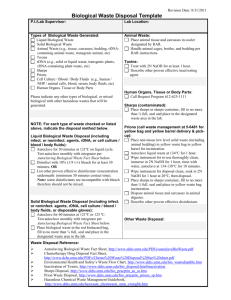
Local Risk Assessment Worksheet for Work with Biological Materials Completed By: Enter text here. Date Completed: Enter text here. Material Description 1. Name or Description of material being handled: Enter text here. 2. Is material considered pathogenic: ☐ Yes ☐ No If Yes… If No… Indicate why it is considered non-pathogenic: Indicate Risk Group: ☐ 1 ☐ 2 ☐ 3 ☐ Material comes from an otherwise How was risk group determined? healthy individual; or, ☐ Pathogen data sheet ☐ Material comes from the environment in ☐ By supplier or other researcher an unaltered state: or ☐ Pathogen risk assessment ☐ Other: Enter text here. ☐ Other: Personnel Factors 1. Vaccine available? ☐Yes - Name of vaccine: ☐No ☐N/A All personnel working with or near any of the above material in use have been: Offered any available vaccinations and the department has a record of the vaccination being received OR declined with counselling. Is a medical surveillance plan in place and documented. Please Describe: ☐ Yes ☐ No ☐ Yes ☐ No Enter text here. Is a Medical Contact Card required? ☐ Yes ☐ No Instructed in signs and symptoms of infection ☐ Yes ☐ No 2. PPE required when working with agent (check all that apply) ☐Face shield ☐safety glasses ☐N-95 ☐ face mask ☐back-closing gown at BSC *Note: lab coat, close toed shoes, and gloves are all mandatory for all microbiological work! 3. Frequency of contact with agent: ☐Routine/daily ☐Weekly ☐Random/monthly /yearly September 2015 Version 2 1 Assessment of Factors Associated with the Specific Work Processes 4. Concentrations and volumes used: Largest single volume used: ☐< 1Litres ☐1-10Litres ☐>10Litres Indicate volume if greater than 10 L Indicate concentrations used (If concentrated enter both before and after concentrations): Enter text here Indicate concentration required to cause infection: ☐ N/A Enter text here 5. Is all work with the active agent done in a BSC? ( not required for CL1 ) ☐Yes ☐No ☐Yes ☐No 6. Is bench work completed on agent? Is material inactivated prior to manipulation? ☐ Yes ☐ No If NO, indicate type of procedures done on open bench (refer to SOPs used): Enter text here How will the hazards of exposure by bench work be mitigated? Enter text here Will any process performed on the bench create aerosols? ☐ Yes ☐ No If YES, indicate how exposure to aerosols will be minimized: Enter text here 7. Will sharps be used? If YES, are you using safety engineered sharps? If not, explain: Enter text here ☐Yes ☐No 8. Processes that increase exposure potential should be identified. Do you use any of the following processes (check all that apply): ☐ Cell sorting ☐ Sonication ☐ Centrifuging in open containers ☐ Blending ☐ Flaming loops ☐ Shaking or vigorous mixing ☐ Grinding ☐ Pipetting ☐ homogenizing ☐ Opening containers with high internal pressures ☐Other procedures that may create an airborne exposure to a pathogen: Enter text here September 2015 Version 2 2 9. Will your experiments involve centrifugation? ☐ Yes ☐ No a. If YES, are sealed rotors, or sealed centrifuge safety cups available for use? ☐ Yes ☐ No b. If NO to “10 a”, do you only use screw –cap non-glass tubes? ☐ Yes ☐ No c. Will you open the tubes in the BSC after centrifuging? ☐ Yes ☐ No If YES, click here to explain how you will protect against exposure. Disinfection and Waste Disposal 10. At what stage of your work will the infectious agent be inactivated or lysed? ☐ N/A Enter text here – note N/A should only be used if there is no infectious agent. 11. Specify disinfectants and decontaminants and decontamination procedures in use: ☐ N/A Disinfectant Contact Time (min) Enter text here. Preparation Frequency Enter text here. Used Against Enter text here. Working Concentration Enter text here. Enter text here Enter text here Enter text here Enter text here Enter text here Enter text here Enter text here Enter text here Enter text here Enter text here Enter text here. 12. Complete the table to identify how biohazardous wastes generated by your research are treated (Any autoclaving and direct disposal requires weekly efficacy logs): Waste Generated and disinfection process Disinfection Parameters Solid waste contaminated with biohazardous material and all ☐Yes ☐ N/A microbial and eukaryotic cell cultures, including broth cultures. Autoclave temp & time ☐No Disposal by: Enter text here oC ☐N/A ☐Biowaste bin (UW Disposal Service) or ☐Autoclaving Enter text here min. ☐Yes ☐No ☐N/A (Sharps) Needle and syringe assemblies will be ☐ N/A Disposed by: ☐Biowaste sharps bin (UW Disposal Service) or ☐Autoclaving Autoclave temp & time Enter text here oC Enter text here min. ☐Yes ☐No ☐N/A Used glass and hard plastic pipettes and Pasteur pipettes will be: Disposed by: ☐Biowaste sharps bin (UW Disposal Service) or ☐Autoclaved and disposed as regular waste ☐ N/A ☐Yes ☐No ☐N/A Liquid waste contaminated with biohazardous material will be Disposal by: ☐Biowaste bin (UW Disposal Service) , ☐Autoclaving, ☐ Chemically Enter Autoclave temp and time OR chemical contact time here. ☐Yes ☐No ☐N/A Other, specify: Enter text here Enter text here September 2015 Version 2 Name of Solution Enter text here Contact time Enter text here hrs 3 Summary: Summarize the SOPs or describe the processes you will be using to minimize risk. Use reference numbers or naming conventions that you use in your lab, and provide a short description of its purpose. These must be made available to the Biosafety Officer upon request. Identify SOPs or Controls used for your work Example – SOP 734 – Purification of xxx by centrifugation…. Enter text here Enter text here Enter text here Identify Controls used for your work Enter text here Safety Office Comments: Supervisor Name: Signature: Date: Biosafety Officer Name: Signature: September 2015 Date: Version 2 4