In the Social Studies folder on the PISD tree, open the Arc View GIS
advertisement
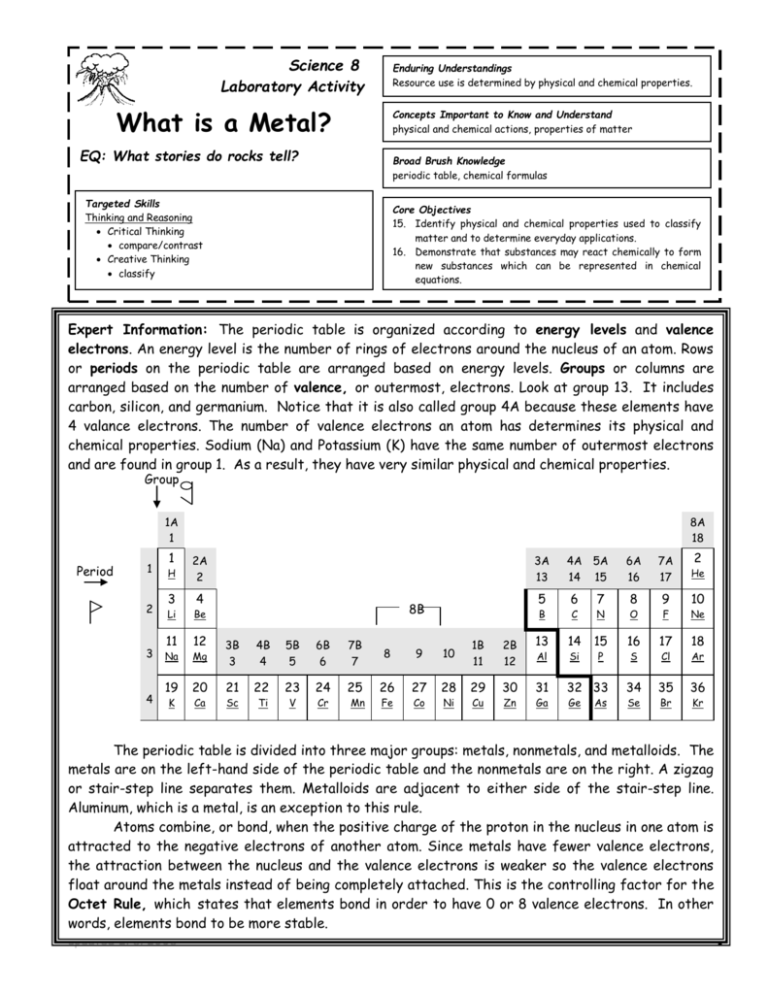
Science 8 Laboratory Activity Enduring Understandings Resource use is determined by physical and chemical properties. What is a Metal? Concepts Important to Know and Understand physical and chemical actions, properties of matter EQ: What stories do rocks tell? Broad Brush Knowledge periodic table, chemical formulas Targeted Skills Thinking and Reasoning Critical Thinking compare/contrast Creative Thinking classify Core Objectives 15. Identify physical and chemical properties used to classify matter and to determine everyday applications. 16. Demonstrate that substances may react chemically to form new substances which can be represented in chemical equations. Expert Information: The periodic table is organized according to energy levels and valence electrons. Examine An energy level isand thechemical number properties of rings ofof electrons around the nucleus of an atom. Purpose physical elements. To determine properties usedRows to or periodsclassify on theelements periodic on table are arranged the periodic table. based on energy levels. Groups or columns are arranged based on the number of valence, or outermost, electrons. Look at group 13. It includes carbon, silicon, and germanium. Notice that it is also called group 4A because these elements have 4 valance electrons. The number of valence electrons an atom has determines its physical and chemical properties. Sodium (Na) and Potassium (K) have the same number of outermost electrons and are found in group 1. As a result, they have very similar physical and chemical properties. Group 1A 1 Period 1 8A 18 2A 2 3A 13 1 H 2 Li 3 Na Mg 12 3B 3 4B 4 5B 5 6B 6 7B 7 8 9 10 1B 11 19 20 21 22 23 24 25 26 27 28 29 4 3 11 K 4 Ca Sc Ti V Cr Mn Fe Co Ni Cu 7A 17 2 He 6 C N O 8 9 F Ne 2B 12 13 14 15 16 17 18 30 31 32 33 34 35 36 B Zn Al Ga Si Ge 7 6A 16 5 8B Be 4A 5A 14 15 P As S Se Cl Br 10 Ar Kr The periodic table is divided into three major groups: metals, nonmetals, and metalloids. The metals are on the left-hand side of the periodic table and the nonmetals are on the right. A zigzag or stair-step line separates them. Metalloids are adjacent to either side of the stair-step line. Aluminum, which is a metal, is an exception to this rule. Atoms combine, or bond, when the positive charge of the proton in the nucleus in one atom is attracted to the negative electrons of another atom. Since metals have fewer valence electrons, the attraction between the nucleus and the valence electrons is weaker so the valence electrons float around the metals instead of being completely attached. This is the controlling factor for the Octet Rule, which states that elements bond in order to have 0 or 8 valence electrons. In other words, elements bond to be more stable. updated 2/6/2006 1 Metals become more stable by getting rid of valence electrons, while nonmetals steal or share electrons to get to 8 valence electrons. Valence electrons are the controlling factors for physical and chemical properties of the atoms as listed in the table below. Properties of Metals and Nonmetals Property Metals Nonmetals Conducts Electricity Does Not Conduct Electricity Conducts Heat Does Not Conduct Heat Luster Shiny Dull Color Silver or Gray Color Earth Tones Strength Malleable Brittle Reacts with Acids Does Not React With Acids Gives off Electrons in Chemical Reactions Steals Electrons in Chemical Reactions Can be Magnetic (4 types of metals are magnetic) Never Magnetic Conductivity Reactivity Magnetism updated 2/6/2006 Reason Electricity needs freely moving electrons to be conducted. Heat is the vibration of the atom. The freely moving electrons allow the heat to be passed at a more rapid rate. The freely moving electrons reflect all the wavelengths of light. The reflection of all the light makes metals appear shiny. The freely moving electrons reflect all the wavelengths of light. The reflection of all the light makes metals appear Silver or Gray The freely moving electrons allow metals to bend instead of break. Acids want extra electrons. Metals electrons float freely around, and are easy to steal The freely moving electrons around the nucleus are easy to steal. Scientists are still not sure how magnetism works. They do know that it involves freely moving electrons, which metals have. 2 Part I Procedure 1. Use the pictures of atoms that are given and a periodic table to answer the questions about the atoms. Data Analysis 1. A. What period is the atom in? B. What group is the atom in? C. What is the name of this atom? D. What other atom would have similar properties to the atom? E. Will this atom conduct electricity well? 2. F. What period is the atom in? G. What group is the atom in? H. What is the name of this atom? I. What other atom would have similar properties to the atom? J. Would this atom react with an acid 3. K. What period is the atom in? L. What group is the atom in? M. What is the name of this atom? N. What other atom would have similar properties to the atom? O. Would this atom be malleable or brittle? updated 2/6/2006 3 PartII Materials Well Plates Metal Samples HCl Alligator Clips with light bulb attached Procedure 1. Using the periodic table, determine the chemical symbol for each element used. Record on the data table. 2. Place the 8 test tubes in the test tube rack. Use forceps or a chemical spoon to place 2 small samples or a few crystals of each element as directed by your teacher. (Do not touch the elements with your hands!) 3. At your lab station, turn the test tubes upside down and empty the samples onto the lab sheet within each labeled circle. 4. Observe color, luster, and any unique observable physical properties for each element. (Do not inhale or taste any substance!) Record observations in the data table. 5. To determine malleability, gently tap the sample element with a small hammer. If the substance flattens without crumbling, it is malleable. If it crumbles, it is brittle. 6. To test each substance for conductivity, use a battery, flashlight bulb, copper wire, battery clips, and a battery holder. Assemble the equipment as indicated in Diagram 1. Connect the two wires to a piece of each element one at a time but do not let the two wires touch. Note the intensity Element of the light to indicate how well the material conducts Diagram 1 electricity. If the bulb does not light at all, the element is not a conductor of electricity. 7. Using a chemical spoon, place one small piece of each element into each test tube. 8. Add 2-3 mL of HCl acid to each test tube. Record all observations in the data table. Watch the reaction for approximately 5 minutes to observe any changes over time in the elements. 9. Discard the materials as directed by your teacher. 10. Clean the test tubes and return them to the test tube rack. 11. Using a chemical spoon, place one small piece of each element into each test tube. 12. Add 2-3 mL of copper chloride solution to each sample. Record all observations in the data table. 13. Watch the reaction for approximately 5 minutes to observe any changes over time in the element. 14. Wash test tubes and turn upside down to dry. 15. Clean up lab station. Data Analysis 1. What accounts for the observed differences between element samples? 2. Based on the lab results, fill in the chart below. Justify your reasons for each classification. Element updated 2/6/2006 Classification Justification 4 Metal/Nonmetal/Metalloid Aluminum Carbon Copper Nickel Magnesium Silicon Sulfur Zinc 3. Using the periodic table provided, circle all the elements used during the lab. What pattern do you see between where the element is placed on the periodic table and its properties? 4. Name 2 characteristics you expect the following elements to have based on where they are located on the periodic table. CalciumBoronChlorine5. Why do some elements that are classified as metals not look metallic? 6. The light bulb lit when connected to some elements, making a complete circuit. Why would the bulb not light when connected to other elements even though everything was connected in the same manner? updated 2/6/2006 5 Data Aluminum Carbon Copper Nickel Magnesium Silicon Sulfur Zinc updated 2/6/2006 6 Physical and Chemical Properties of Specific Elements Element Chemical Symbol Color Luster Other Physical Properties Malleable or Brittle Reaction to HCl acid Reaction to Copper Chloride Conductivity Aluminum Carbon Copper Nickel Magnesium Silicon Sulfur Zinc Data Table updated 2/6/2006 7







