Phase Diagram Worksheet: Chemistry Practice
advertisement

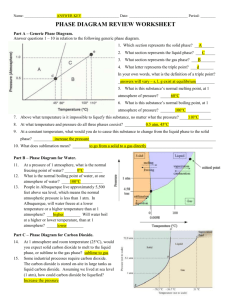
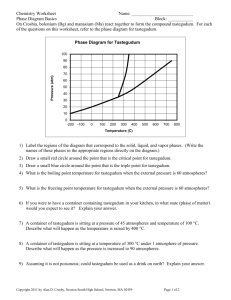
Name: _______________________________ Chemistry 22 Phase Diagram Worksheet Answer the questions below in relation to the following phase diagram. 1. Which section represents the solid phase? _______ 2. What section represents the liquid phase? _______ 3. What section represents the gas phase? ________ 4. What letter represents the triple point? ________ 5. What’s so special about the triple point? _________________________________________________________________________ _________________________________________________________________________ 6. Different points on a phase diagram are often described as “at equilibrium.” What does this term mean? (You might need to look it up!) _________________________________________________________________________ _________________________________________________________________________ 7. What is this substance’s melting point at 1 atmosphere of pressure? _________ 8. What is this substance’s boiling point at 1 atmosphere of pressure? _________ For questions 9 – 11, refer to the phase diagram below of a pure substance. (A) Sublimation (C) Vaporization (B) Condensation (D) Melting (E) Deposition 9. If the pressure decreases from 1.5 to 0.5 atmospheres at a constant temperature of 50˚C, which of the processes occurs? ____________________________ 10. If the temperature increases from 10°C to 50°C at a constant pressure of 0.5 atmospheres, which of the processes occurs? ____________________________ 11. If the temperature decreases from 110°C to 40°C at a constant pressure of 1.1 atmospheres, which of the processes occurs? ____________________________ 12. What is unique about the phase diagram for water? Explain. _________________________________________________________________________ _________________________________________________________________________