Density Calculation Worksheet There are physical characteristics of
advertisement

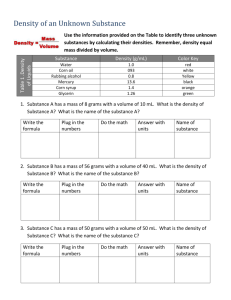
Density Calculation Worksheet There are physical characteristics of a substance that help identify the substance. One of these characteristics is density. Density is defined as mass per unit volume. Density is calculated by dividing the mass of an object by its volume. This is shown in equation form, as follows: density = mass volume Depending whether the material is a solid or a liquid, the units for density may be g/cm 3 or g/mL. Solids: Density = mass (g) Volume (cm3) (g/cm3) Liquids: Density = mass (g) Volume (mL) (g/mL) Remember that volume can be found by: V=LxWxH Measuring it with a graduated cylinder Water displacement (Water in graduated cylinder plus object, and finding the difference in water level) Using Cross multiplication When the density is given but either the mass or volume is missing, then use cross multiplication for solving for m or v: Example Cross multiplication can help speed up a solution to isolate the variable that you are trying to find. Like in this example: The density of an object is 2 g/cm3 and its volume is 8 cm3. Find its mass. 1) Plug in the numbers in the density equation d=m 2 g/cm3 = 8 cm3 2= 8 v m m 2) Solve for m by Cross multiplying: 2= 8 2m = 8 m 3) Solve the equation: m = 8/2 = 4, don’t forget to write the units! m=4g Practice Problems – Do your work on a different sheet. Do not forget to include the right units. ROUND YOUR ANSWER to the Tenth value whenever applicable. 1) A block of aluminum occupies a volume of 15.0 mL and has a mass of 40.5 g. What is its density? Write Density Equation: Plug in Numbers: Solve Equation: 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder has a mass of 306.0 g. From this information, calculate the density of mercury. Write Density Equation: Plug in Numbers: Solve Equation: 3) What is the mass of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL. Write Density Equation: Plug in Numbers: Solve Equation: 4) A rectangular block of copper metal has a mass of 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper? Write Density Equation: Plug in Numbers: Solve Equation: 5) A flask that has a mass of 345.8 g is filled with 225 mL of carbon tetrachloride. The mass of the flask and carbon tetrachloride is found to be 703.55 g. From this information, calculate the density of carbon tetrachloride. Write Density Equation: Plug in Numbers: Solve Equation: 6) Calculate the density of sulfuric acid if 35.4 mL of the acid has a mass of 65.14 g. Write Density Equation: Plug in Numbers: Solve Equation: 7) Find the mass of 250.0 mL of benzene. The density of benzene is 0.8765 g/mL. Write Density Equation: Plug in Numbers: Solve Equation: 8) A block of lead has dimensions of 4.50 cm by 5.20 cm by 6.00 cm. The block has a mass of 1587 g. From this information, calculate the density of lead. Write Density Equation: Plug in Numbers: Solve Equation: 9) 28.5 g of iron shot is added to a graduated cylinder containing 45.50 mL of water. The water level rises to the 49.10 mL mark, from this information, calculate the density of iron. Write Density Equation: Plug in Numbers: Solve Equation: 10) What volume of silver metal will have a mass of exactly 2500.0 g. The density of silver is 10.5 g/cm3. Write Density Equation: Plug in Numbers: Solve Equation: