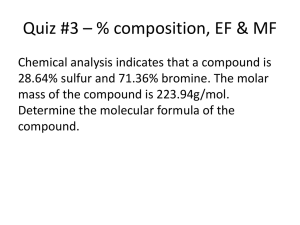
Chapter 7 Ions and Naming
advertisement

Chem 1 Ions and Naming Practice Quiz 1. Which of the following elements DO NOT form diatomic molecules? a) hydrogen b) helium c) nitrogen d) chlorine e) iodine 2. The result of changing a subscript in a correctly written compound is to a) change the the compound into an element b) change the charges on the other ions in the compound c) change the formula so that it no longer represents the compound it previously did d) have no effect on the formula 3. How many total atoms are in the following compound : Ca(HCO3)2 a) 4 b) 6 c) 8 d) 11 4. What is the name of the compound KBr? a) potassium I bromine b) potassium bromine c) potassium monobromide d) potassium bromide 5. What is the name of the following compound? K2S a) potassium sulfide b) dipotassium disulfide c) potassium disulfide d) potassium II sulfide e) potassium I sulfide 6. What is the formula for the compound magnesium chloride? a) MgCl b) MgClO3 c) Mg2Cl d) MgCl2 e) MgCO3 7. What is the formula for the compound aluminum sulfide? a) AlS b) AlS3 c) Al2S3 d) Al3S2 e) AlSO4 8. What is the formula for the compound Nickel II chloride? a) NiCl b) Ni2Cl c) Ni2Cl2 d) NiCl2 9. What is the name of the compound MnF2? a) Magnesium fluoride b) Manganese fluoride c) Manganese difluoride d) Manganese I fluoride e) Manganese II fluoride 10. What is name of the compound Cd3N2? a) Cadmium nitrate b) Cadmium II nitrate c) Cadmium II nitride d) Cadmium III nitride e) Tricadmium dinitride 11. Write the formula for the compound silver I sulfide. a) AgS b) AgS2 c) Ag2S d) Ag2S3 e) Ag2S2 12. What is the formula for carbon disulfide? a) CS b) CS2 c) C2S d) C2S2 e) C(SO4)2 13. What is the formula for diphosphorus pentoxide? a) PO b) P2O5 c) P5O2 d) P2O6 e) P2O4 14. What is the name of the compound IBr? a) Iodine bromide b) Iodine I bromide c) monoiodine monobromide d) Iodine monobromide e) Iodine bromine 15. What is the formula for the compound silicon dioxide? a) SiO2 b) SO2 c) SiO d) S2O e) Si2O2 16. What is the name of the compound As2O5? a) Arsenic Oxide b) Arsenic II Oxide c) Arsenic III Oxide d) Diarsenic Pentoxide e) Arsenic Pentoxide 17. Name the following compound: IF7. a) Iodine Fluoride b) Iodine I Fluoride c) Iodine Heptafluoride d) Iodine Hexafluoride e) Monoiodine Heptafluoride 18. What is the name for the compound CaH2? a) Calcium Hydroxide b) Calcium dihydroxide c) Calcium dihydride d) Monocalcium hydride e) Calcium hydride 19. What is the name of the compound LiNO 3? a) Lithium nitrate b) Lithium mononitrate c) Monolithium mononitrate d) Lithium I nitrate e) Lithium nitride 20. What is the name of the compound Cu2CO3? a) Copper carbonate b) Dicopper carbonate c) Copper II carbonate d) Copper I carbonate e) Dicopper monocarbonate 21. Name the following compound Ca3(PO4)2. a) Calcium phosphate b) Calcium II phosphate c) Calcium III phosphate d) Tricalcium diphosphate e) Calcium phosphide 22. Name the following compound NiCO3. a) Nickel carbonate b) Nickel I carbonate c) Nickel II carbonate d) Nickel monocarbonate e) Mononickel monocarbonate 23. Name the following compound NH3. a) Nitrogen III hydride b) Nitrogen trihydride c) Ammonia d) Ammonium e) Mononitrogen trihydride 24. How many electrons does aluminum ion have? a) 10 b) 13 c) 15 d) 16 e) 18 25. What charge do the ions of all the halogens have? a) +1 b) -1 c) +2 d) -2 e) you can't tell since they form various ions 26. Positively charged ions are called a) charges b) cations c) anions d) diatomic e) polyatomic 27. What charged ion will the element Cobalt usually form? a) +1 b) +2 c) -1 d) -2 e) can't tell since it may form more than one ion 28. Potassium and Sulfur will form which of the following compounds? a) KS b) K2S c) KS2 d) K3S e) Not enough information since potassium may form more than one ion. 29. What compound will the elements Calcium and Nitrogen form? a) CaNi b) CaN c) Ca2N d) Ca3N2 e) CaN3 30. Which of the following statements is FALSE? a) Metals tend to lose electrons in chemical reactions b) Nonmetals can form anions c) Ions form when an element gains or loses protons d) When an element forms an ion, it must gain or lose the same number of protons as electrons e) C and D ----------Key---------1. (b) 2. (c) 3. (d) 4. (d) 5. (a) 6. (d) 7. (c) 8. (d) 9. (e) 10. (c) 11. (c) 12. (b) 13. (b) 14. (d) 15. (a) 16. (d) 17. (c) 18. (e) 19. (a) 20. (d) 21. (a) 22. (c) 23. (c) 24. (a) 25. (b) 26. (b) 27. (e) 28. (b) 29. (d) 30. (e)