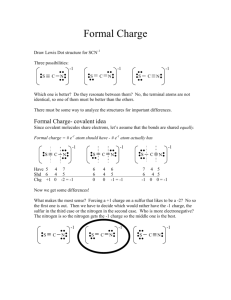
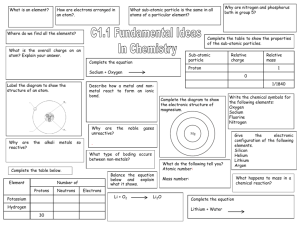
Midterm Review
advertisement

Midterm Review 1. Iodine-131 is a radioactive isotope with a half-life of 8 days. How many grams of a 64 g sample of iodine-131 will remain at the end of 24 days? 2. The coefficients necessary to correctly balance the equation below are – ___Mg(OH)2 + ___HCL ___MgCl2 + ___H2O 3. Which of these elements has the smallest atomic radius? a. Beryllium (Be) b. Oxygen (O) c. Sodium (Na) d. Sulfur (S) 4. At atom contains 70 protons, 70 electrons, and 99 neutrons. What is the mass number? 5. Sodium chloride conducts electricity when dissolved in water. What type of bond is present in NaCl? a. Nonpolar covalent b. Polar covalent c. Hydrogen d. Ionic 6. A sodium atom has an electron configuration of 1s22s22p63s1. If the sodium atom becomes ionized, its new electron configuration will be the same as which element? a. Lithium b. Neon c. Magnesium d. Potassium 7. According to the periodic table, Mg will most likely react with elements in which of these groups? a. 1 b. 3 c. 17 d. 18 8. Which of these compounds is most likely to contain an ionic bond? a. H2 b. SO2 c. CH4 d. CaCl2 9. The chemical reaction shown below is an example of – Cu + 2AgNO3 Cu(NO3)2 + 2Ag a. b. c. d. single-replacement reaction synthesis reaction decomposition reaction double-replacement reaction 10. Which element is a noble gas? a. Fluorine (F) b. Hydrogen (H) c. Nitrogen (N) d. Xenon (Xe) 11. What is the name of NH4OH? a. Ammonium hydroxide b. Nitrogen oxygen hydride c. Nitrogen hydroxide d. Ammonium oxygen hydride 12. What is the correct name for the compound P4O6? a. Phosphoric acid b. Phosphorus oxide c. Phosphorus(IV) oxide d. Tetraphosphorus hexoxide 13. The accepted value for the specific heat of aluminum is 0.897 J/gC. Which of the following sets of specific heat values for aluminum, calculated from a prior experiment, has the greatest accuracy and precision? a. 0.847 J/gC, 0.847 J/gC, 0.848 J/gC b. 0.896 J/gC, 0.899 J/gC, 0.896 J/gC c. 0.897 J/gC, 1.04 J/gC, 1.03 J/gC d. 0.936 J/gC, 0.876 J/gC, 0.879 J/gC 14. What is the chemical formula for iron(II) phosphide? 15. What are the numbers of protons, neutrons, and electrons in an isotope of titanium with a mass number of 50? 16. How many protons are in an atoms represented by 220Ra? a. 88 b. 132 c. 220 d. 308 17. A chloride ion (Cl-) has the same number of electrons as a neutral atom of – a. fluorine b. sulfur c. argon d. bromine 18. What are shared in covalent bonds? a. Cations b. Protons c. Electrons d. Anions 19. Silicon has three naturally occurring isotopes: 28Si occurs 28%, 29Si occurs 29%, and 30 occurs 43%. Calculate the average atomic mass. 20. A student calculated the density of metal to be 3.55 g/mL. The accepted density of the metal is 3.78 g/mL. What is the student’s percent error? 21. A compound has an empirical formula of N2O3. The molecular formula has a molar mass of 304 g/mole. What is the molecular formula?