Homework 01 for Lectures 1
advertisement

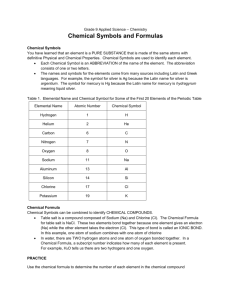
Practice 1 Lectures 1- 3 Name _____________________ Use this to review lectures 1 through 3. 1. For each of the crystal systems, sketch the axes with the correct axis names in their correct positions, and write down the length rules and angle rules. Use a separate piece of paper, and attach it to this. 2. Explain the four symmetry elements: rotation, reflection, center of symmetry, and rotation with inversion. Use simple sketches to explain our answer with 2-sided, asymmetrical motifs such as these: Use a separate piece of paper, and attach it to this. 3. A crystal face intersects the axes at a = 3, b = 3, and c = 3/2. Write down the Miller Index for this face. 4. a. Define, and sketch simple examples of, the four principle forms: pedion, pinacoid, prism and pyramid. b. Also define a zone. Use a separate piece of paper, and attach it to this. 5. Plot the (101) face on the Wulff net on the right. Latitude Phi = 45 North and Longitude rho = 0 East 6. What is the electron configuration of a. a neutral atom of Sodium, symbol Na, element 11? b. The common ion of Sodium, Na+ c. How many protons does neutral Sodium have? d. How many protons does the Na+ ion have? 7. In covalent bonding, atoms share electrons. True or False? 8. In ionic bonding, atoms lose or gain electrons. True or False? 9. What is the difference in electronegativity between a. Potassium, symbol K, and Chlorine, symbol Cl? ___ b. What type of bond would KCl (Sylvite) have? Choose: Ionic / Covalent