Stoichiometry Mass-Mass Problem Worksheet
advertisement
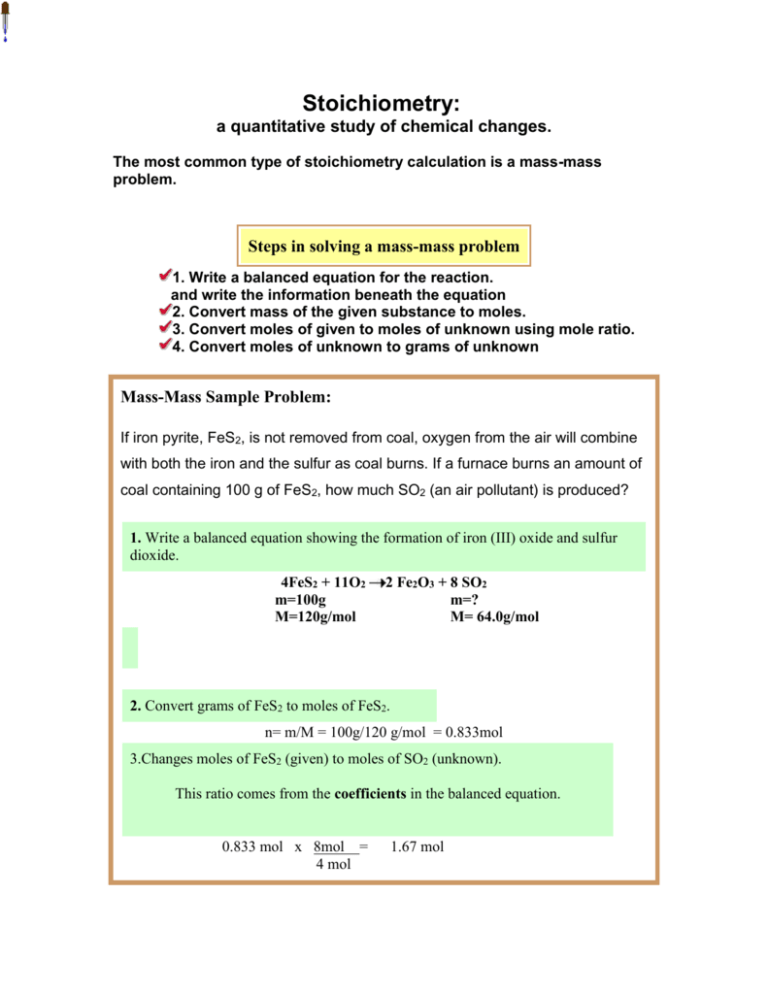
Stoichiometry: a quantitative study of chemical changes. The most common type of stoichiometry calculation is a mass-mass problem. Steps in solving a mass-mass problem 1. Write a balanced equation for the reaction. and write the information beneath the equation 2. Convert mass of the given substance to moles. 3. Convert moles of given to moles of unknown using mole ratio. 4. Convert moles of unknown to grams of unknown Mass-Mass Sample Problem: If iron pyrite, FeS2, is not removed from coal, oxygen from the air will combine with both the iron and the sulfur as coal burns. If a furnace burns an amount of coal containing 100 g of FeS2, how much SO2 (an air pollutant) is produced? 1. Write a balanced equation showing the formation of iron (III) oxide and sulfur dioxide. 4FeS2 + 11O2 m=100g M=120g/mol 2 Fe2O3 + 8 SO2 m=? M= 64.0g/mol 2. Convert grams of FeS2 to moles of FeS2. n= m/M = 100g/120 g/mol = 0.833mol 3.Changes moles of FeS2 (given) to moles of SO2 (unknown). This ratio comes from the coefficients in the balanced equation. 0.833 mol x 8mol = 4 mol 1.67 mol 4 Convert moles of SO2 to grams of SO2 . m =n x M = 1.67 mol x 64.00 g/mol = 107 g of SO2 All units have been canceled except for grams of SO2(unknown). The problem has been solved. 107 grams of SO2 will be produced.









