RING OUZEL BREEDING SUCCESS - GLEN CLUNIE 1998
advertisement

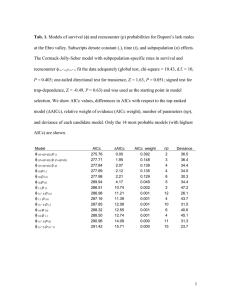
1 Supporting Information for: Characterising demographic variation and contributions to population growth rate in a declining population Innes M.W. Sim, Graham W. Rebecca, Sonja Ludwig, Murray C. Grant & Jane M. Reid 2 Table S1 Sample sizes for the variables shown in Fig.3. Rate codes are defined in Table 1. 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 All years Early laying date 23 25 32 31 30 21 23 20 15 22 21 15 278 Late laying date 16 14 30 24 22 21 16 17 17 11 10 16 214 Early clutch size 8 22 23 20 17 17 22 18 14 17 18 12 208 Late clutch size 13 8 19 13 22 17 10 13 13 11 11 11 161 Early fledged brood size 27 26 23 23 27 16 21 17 11 15 13 12 231 Late fledged brood size 13 12 21 20 18 17 12 13 12 9 7 14 168 Early DNSR 8 18 24 18 16 19 21 19 13 15 16 11 198 Late DNSR 10 6 18 12 20 16 9 10 13 10 12 9 145 Ce 23 32 43 41 39 38 39 24 26 26 28 21 380 Cl 17 16 29 27 26 24 21 18 16 14 17 13 238 RSe 8 18 23 18 16 16 21 17 11 15 13 11 187 RSl 10 6 18 12 18 16 9 10 12 9 7 9 136 Overall RS 8 6 18 12 16 16 9 10 11 9 7 9 131 3 Table S2 Models used to estimate daily nest survival rates (DNSR). ne, nl, and y indicate early-nest, latenest and year dependence in nest survival () probability respectively. ‘+trend’ and ‘*y’ indicate a linear change across years, and year-dependent effects, respectively. AIC scores corrected for small sample size (AICc), the AIC difference between the current and best model (AICc) and the relative weight of each AICc value (AICc weight) are presented. Analyses used nest histories from 198 early and 145 late nests. The best supported model is indicated in bold. Model AICc AICc AICc weight Parameters Deviance (.) 849.8 0.0 0.398 1 847.8 (ne, nl) 850.8 1.0 0.238 2 846.8 (ne, nl+trend) 851.2 1.5 0.193 3 845.2 (ne+trend, nl) 852.6 2.8 0.098 3 846.6 (ne+trend, nl+trend) 853.7 4.0 0.055 4 845.7 (ne*y, nl) 856.0 6.2 0.018 13 830.0 (ne, nl*y) 864.7 14.9 0.000 13 838.7 (ne*y, nl*y) 870.0 20.2 0.000 24 821.8 4 Table S3 Capture-mark-recapture models used to estimate adult annual apparent survival probabilities (ad). m, f, s, and y indicate male, female, sex and year dependence in resighting (p) and ad respectively. ‘+trend’, ‘*y’ and ‘*rand’ indicate a linear change across years, year-dependent effects and random effects model fitted to year, respectively. Analyses used encounter histories from 221 individuals (103 males and 118 females). The best supported model is indicated in bold. Model AICc AICc AICc weight Parameters Deviance (s)p(.) 511.5 0.0 0.329 3 86.1 (m+trend, f)p(.) 512.7 1.3 0.175 4 85.3 (m, f*rand)p(.) 512.8 1.3 0.169 6.9 79.3 (s+trend)p(.) 513.3 1.8 0.133 4 85.8 (m, f+trend)p(.) 513.5 2.0 0.120 4 86.0 (s*trend)p(.) 514.8 3.3 0.063 5 85.3 (m, f*y)p(.) 520.0 8.5 0.005 12 75.8 (s)p(y) 520.4 8.9 0.004 12 76.2 (y)p(.) 522.4 10.9 0.001 11 80.3 (s*y, f*rand)p(.) 525.3 13.8 0.000 15.9 72.6 (s*y)p (.) 533.1 21.6 0.000 21 69.0 (s*y)p(y) 547.4 35.9 0.000 29 64.9 (s*y)p(s*y) 567.7 56.2 0.000 38 63.3 5 Table S4 Known fate models used to estimate post-fledging weekly survival probabilities (f). be, bl and w indicate early brood, late brood and weekly dependence in f respectively. ‘*w’ indicates a weekdependent effect on survival. The best supported model is indicated in bold. Model AICc AICc AICc weight Parameters Deviance (be, bl) 316.6 0.0 0.405 2 14.2 (be*w, bl) 317.5 0.9 0.253 6 6.9 (be, bl*w) 317.9 1.3 0.214 6 7.3 (be*w, bl*w) 319.0 2.4 0.120 10 0.0 (.) 325.4 8.8 0.005 1 25.0 (w) 326.9 10.3 0.002 5 18.4 6 Table S5 Capture-mark-recapture models used to estimate first-year annual apparent survival probabilities (1). be, bl, ae, al and y indicate early-brood, late-brood, early-brood adult (1+years), late-brood adult (1+years) and year dependence in re-sighting (p) and 1 respectively. Adult survival in this model was included as a nuisance variable only. ‘+trend’ and ‘*y’ indicate a linear change across years, and year-dependent effects, respectively. Analyses used encounter histories from 1386 individuals (799 early and 587 late brood chicks). The best supported model is indicated in bold. Model AICc AICc AICc weight Parameters Deviance (b+trend, a)p(.) 599.8 0.0 0.412 4 79.1 (be, bl, a)p(.) 600.4 0.6 0.305 4 79.7 (b*trend, a)p(.) 601.9 2.0 0.151 5 79.1 (be, bl, ae, al)p(.) 602.3 2.5 0.118 5 79.6 (b, a)p(.) 607.1 7.3 0.011 3 88.3 (be*y, bl, a)p(.) 610.0 10.2 0.003 14 69.0 (be, bl*y, ae, al)p(.) 615.1 15.3 0.000 15 72.0 (be*y, bl*y, a)p(.) 623.0 23.2 0.000 24 61.5 (be*y, bl*y, ae, al)p(.) 625.0 25.1 0.000 25 61.4 (be*y, bl*y, ae, al)p(y) 644.8 45.0 0.000 35 60.3 (be*y, bl*y)p(y) 791.3 191.5 0.000 32 213.2 (be*y, bl*y)p(b*y) 810.3 210.4 0.000 41 213.2 7 Table S6 Comparison of mean estimates of reproductive success (RS) and survival for ring ouzels in Glen Clunie with those from other studies on ring ouzels and other related species. Figures separated by ‘/’ are for early- and late-nests respectively, while figures separated by ‘-‘ indicate the range in values from various studies. Clutch Fledged size brood size ONSR RS f 1 week f 5 weeks ad References Ring ouzel 4.0/4.0 3.6/3.7 0.65/0.57 2.3/2.1 0.88/0.74 0.57/0.22 0.42 this study Ring ouzel 3.9-4.2 3.4-4.0 0.41-0.68 1.6-2.5 - - - 1 Blackbird 3.9-4.1 3.4-3.5 0.14-0.55 1.9 - - 0.65 2-5 Song thrush 4.2/3.8 3.9/3.2 0.19-0.44 0.8-1.6 0.77 0.30 0.56-0.63 4-9 Wood thrush 3.3/2.6 2.0-3.3 0.26-0.65 0.6-1.9 0.71-0.93 0.43 0.58-0.64 10-20 Ovenbird 2.8-4.9 4.3-4.7 0.26-0.69 1.4-2.9 0.68 0.56 0.52-0.60 21-23 Swainson’s thrush - - - - - 0.52 0.56-0.57 24-26 8 References 1. Burfield, I. (2002) The breeding ecology and conservation of the ring ouzel Turdus torquatus in Britain. Unpublished PhD Thesis. University of Cambridge. 2. Hatchwell, B.J., Chamberlain, D.E. & Perrins, C.M. (1996) The reproductive success of blackbirds Turdus merula in relation to habitat structure and choice of nest site. Ibis, 138, 256-262. 3. BTO Bird Facts. http://www.bto.org/birdfacts/index.htm. 4. Paradis, E., Braille, S.R., Sutherland, W.J. & Gregory, R.D. (1998) Patterns of natal and breeding dispersal in birds. Journal of Animal Ecology, 67, 518-536. 5. Siriwardena, G.M., Baillie, S.R. & Wilson, J.D. (1998) Variation in the survival rates of some British passerines with respect to their population trends on farmland. Bird Study, 45, 276-292. 6. Thomson, D.L. & Cotton, P.A. (2000) Understanding the decline of the British population of song thrushes Turdus philomelos. Ecology and Conservation of Lowland Farmland Birds (eds N.J. Aebischer, A.D. Evans, P.V. Grice & J.A. Vickery), pp. 151–155. British Ornithologists’ Union, Tring, UK. 7. Kelleher, K.M. & O’Halloran, J. (2007) Influence of nesting habitat on breeding Song Thrushes Turdus philomelos. Bird Study, 54, 221-229. 8. Robinson, R.A., Green, R.E., Baillie, S.R., Peach, W.J. & Thomson, D.L. (2004) Demographic mechanisms of the population decline of the song thrush Turdus philomelos in Britain. Journal of Animal Ecology, 73, 670–682. 9. Payevsky, V.A. & Vysotsky, V.G. (2003) Migratory song thrushes Turdus philomelos hunted in Europe: survival rates and other demographic parameters. Avian Science, 3, 13-20. 9 10. Brown, W.P. & Roth, R.R. (2002) Temporal patterns of fitness and survival in the Wood Thrush. Ecology, 83, 958-969. 11. Simons, T.R., Farnsworth, G.L. & Shriner, S.A. (2000) Evaluating Great Smoky Mountains National Park as a population source for the Wood Thrush. Conservation Biology, 14, 1133-1144. 12. Donovan, T.M., Thompson, F.R., Faaborg, J. & Probst, J.R. (1995) Reproductive success of migratory birds in habitat sources and sinks. Conservation Biology, 9, 1380-1395. 13. Kaiser, S.A. & Lindell, C.A.. 2007. Effects of distance to edge and edge type on nestling growth and nest survival in the wood thrush. The Condor, 109, 288-303. 14. Fauth, P.T. (2001) Wood thrush populations are not all sinks in the agricultural Midwestern United States. Conservation Biology, 15, 523-527. 15. Fauth, P.T. (2000) Reproductive success of Wood Thrushes in forest fragments in northern Indiana. The Auk, 117, 194-204. 16. Powell, L. A., Lang, J.D., Krementz, D.G. & Conroy, M.J. (2005) Use of radio-telemetry to reduce bias in nest searching. Journal of Field Ornithology, 76, 274-278. 17. Anders, A.D., Dearborn, D.C., Faaborg, J. & Thompson, F.R. (1997) Juvenile survival in a population of neotropical migrant birds. Conservation Biology, 11, 698-707. 18. Schmidt, K.A., Rush, S.A. & Ostfeld, R.S. (2008) Wood thrush nest success and post-fledging survival across a temporal pulse of small mammal abundance in an oak forest. Journal of Animal Ecology, 77, 830-837. 10 19. Savidge, I.R. & Davis, D.E. (1974) Survival of some common passerines in a Pennsylvania woodlot. Bird-Banding, 45, 152-155. 20. Roth, R.R. & Johnson, R.K. (1993) Long-term dynamics of a Wood Thrush population breeding in a forest fragment. The Auk, 110, 37-48. 21. Flaspohler, D.J., Temple, S.A. & Rosenfield, R.N. (2001) Effects of forest edges on Ovenbird demography in a managed forest landscape. Conservation Biology, 15, 173-183. 22. Porneluzi, P.A. & Faaborg, J. (1999) Season-long fecundity, survival, and viability of ovenbirds in fragmented and unfragmented landscapes. Conservation Biology, 13, 1151-1161. 23. Bayne, E.M. & Hobson, K.A. (2002) Annual survival of adult American Redstarts and Ovenbirds in the southern Boreal forest. Wilson Bulletin, 114, 358–367. 24. White, J.D., Gardali, T., Thompson, F.R. & Faaborg, J. (2005) Resource selection by juvenile Swainson’s Thrushes during the postfledging period. The Condor, 107, 388-401. 25. Gardali, T., Barton, D.C., White, J.D. & Geupel, G. (2003) Juvenile and adult survival of Swainson’s thrush (Catharus ustulatus) in coastal California: annual estimates using capture-recapture analyses. The Auk, 120, 1188-1194. 26. Nichols, J. D., Noon, B.R., Stokes, S.L. & Hines, J.E. (1981) Remarks on the use of mark-recapture methodology in estimating avian population size. Studies in Avian Biology, 6, 121–136.