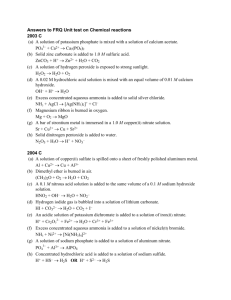
EQUATION PRODUCTS PRACTICE
advertisement

EQUATION PRODUCTS PRACTICE Give the formulas to show the reactants and the products of the following chemical reactions. Each of the reactions occurs in aqueous solution unless otherwise indicated. Represent substances in solution as ions if the substance is extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction. In all cases a reaction occurs. You need not balance. (a) Ethanol is burned in oxygen. (b) Solid barium oxide is added to distilled water. (c) Chlorine gas is bubbled into cold, dilute solution of potassium hydroxide. (d) A solution of iron(II) nitrate is exposed to air for an extended period of time. (e) Excess concentrated sulfuric acid is added to solid calcium phosphate. (f) Hydrogen sulfide gas is bubbled into a solution of mercury(II) chloride. (g) Solid calcium hydride is added to distilled water. (h) A bar of zinc metal is immersed in a solution of copper(II) sulfate. (i) Solid calcium carbonate is strongly heated. (j) A piece of nickel metal is immersed in a solution of copper(II) nitrate. (k) Equal volumes of equimolar solutions of disodium hydrogen phosphate and hydrochloric acid are mixed. (l) Chlorine gas is bubbled into a solution of sodium bromide. (m) Ammonia gas is bubbled into a solution of ethanoic (acetic) acid. (n) Solid ammonia carbonate is added to a saturated solution of hydroxide. (o) Drops of liquid dinitrogen trioxide are added to distilled water. barium (p) Solutions of potassium permanganate and sodium oxalate are mixed. (q) Magnesium metal is burned in nitrogen gas. (r) Sulfur dioxide gas is passed over solid calcium oxide. (s) Lead foil is immersed in silver nitrate solution. (t) A solution of ammonium sulfate is added to a saturated solution of barium Acetic acid solution of ammonium sulfate is added to a solution of sodium hydroxide. (u) hydrogen carbonate. (v) Solid sodium dichromate is added to an acidified solution of sodium (w) Solid calcium oxide is exposed to a steam of carbon dioxide gas. (x) Dinitrogen trioxide gas is bubbled into water. (y) Sodium hydrogen carbonate is dissolved in water. (z) Pellets of lead are dropped into hot sulfuric acid. (aa) A drop of potassium thiocyanate is added to a solution of iron(III) iodide. chloride bb) Solutions of zinc sulfate and sodium phosphate are mixed. cc) Solutions of silver nitrate and lithium bromide are mixed. dd) A stream of chlorine gas is passed through a solution of cold, dilute hydroxide. ee) Excess hydrochloric acid solution is added to a solution of potassium ff) A solution of tin (II) chloride is added to an acidified solution of permanganate. sodium sulfite. potassium gg) A solution of ammonium thiocyanate is added to a solution of iron (III) chloride. hh) Samples of boron trichloride gas and ammonia gas are mixed. ii) Carbon disulfide vapor is burned in excess oxygen. jj) A strip of copper is immersed in dilute nitric acid. kk) Potassium permanganate solution is added to an acidic solution of peroxide. hydrogen ll) Concentrated hydrochloric acid is aded to solid manganese(II) sulfide mm) Excess chlorine gas is passed over hot iron filings. nn) Water is added to a sample of solid magnesium nitride. oo) Excess sulfur dioxide gas is bubbled through a dilute solution of hydroxide. pp) Excess concentrated ammonia solution is added to a suspension of qq) Solutions of tri-potassium phosphate and zinc nitrate are mixed. potassium silver chloride. EQUATION PRODUCTS ANSWERS (A) C2H50H + O2 ------> CO2 + H2O (B) BaO + H2O ------> Ba2+ + OH- (C) Cl2 + OH- ------> Cl- + ClO- (+H2O) (D) Fe2+ + O2 (+H2O) ------> Fe2O3 or FeO(OH) or Fe(OH)3 (E) H2SO4 + Ca3(PO4)2 ------> H3PO4 + CaSO4 (F) H2S + Hg2+ ------> HgS + H+ or H2S + HgCl2 ------> HgS + H+ + Cl- (G) CaH2 + H2O ------> Ca2+ + OH- (or Ca(OH)2) + H2 (H) Zn + Cu2+ ------> Zn2+ + Cu (I) CaCO3 ------> CaO + CO2 (J) Ni + Cu2+ ------> Ni2+ + Cu (K) HPO42- + H+ ------> H2PO4- (L) Cl2 + Br- ------> Cl- + Br2 (M) NH3 + HC2H3O2 ------> C2H3O2- + NH4+ (N) (NH4)2CO3 + Ba2+ + OH- ------> NH3 + BaCO3 + H2O (O) N2O3 + H20 ------> HNO2 (P) MnO4- + C2O42- ------> MnO2 + CO2 (Q) Mg + N2 ------> Mg3N2 (R) (S) SO2 + CaO ------> CaSO3 (Ca2+ + SO32- or Ca2+ + SO32-) Pb + Ag+ ------> Pb2+ + Ag (T) NH4+ + SO42- + Ba2+ + OH- ------> BaSO4 + NH3 + H2O (U) CH3COOH + HCO3- ------> CH3COO- + CO2 + H2O (V) Na2Cr2O7 + I- ------> Cr3+ + I2 (W) CaO + CO2 ------> CaCO3 (X) N2O3 + H2O ------> HNO2 (Y) NaHCO3 + H2O ------> OH- + H2CO3 + (Na+) (Z) (aa) Pb + H+ + SO42- (or HSO4-) ------> PbSO4 + H2 Fe3+ + SCN- ------> FeSCN2+ (bb) Zn2+ + PO43- ------> Zn3(PO4)2 (cc) Ag+ + Br- --------> AgBr Cl2 + 2OH- ------> OCl- + Cl- +H2O (dd) (ee) H+ + SO32- -------> H2O + SO2 or H2SO3 (ff) Sn2+ + H+ + MnO4- -------> Sn4+ +Mn2+ +H2O (gg) Fe3+ + SCN- ------> FeSCN2+ (hh) BCl3 + NH3 -------> Cl3BNH3 (ii) CS2 + O2 --------> CO2 + SO2 or SO3 (jj) Cu + H+ + NO3- ------> Cu2+ + NO + H2O (kk) MnO4- + H2O2 ------> Mn2+ + O2 + H2O (ll) H+ + MnS -------> H2S + Mn2+ (mm) Fe + Cl2 -------> FeCl3 (nn) Mg3N2 + H2O --------> Mg(OH)2 + NH3 (oo) SO2 + OH- --------> HSO3- (pp) AgCl + NH3 -------> Ag(NH3)2+ + Cl- (qq) Zn2+ + PO43- --------> Zn3(PO4)2