What mass of copper oxide can we make from copper carbonate
advertisement

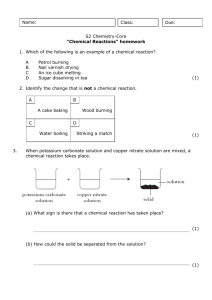
What mass of copper oxide can we make from copper carbonate? Introduction When you heat copper carbonate you get copper oxide and carbon dioxide. We can do an experiment to find out how much copper oxide we can make from a known mass of copper carbonate. We can then compare our result with the calculated value The equation is: CuCO3 CuO + CO2 SAFETY SPECS MUST BE WORN Procedure 1. Find the mass of your empty weighing boat 2. Find the mass of a weighing boat plus three spatulas of copper carbonate. 3. Transfer these to a boiling tube and heat strongly until all the powder is black copper oxide. 4. Let it cool and find the mass of the black copper oxide plus the weighing boat Questions 1. Work out what mass of copper oxide you should have produced in theory from your mass of copper carbonate CuCO3 CuO + CO2 2. How does this compare with your experiment? Can you explain any differences? 3. What is the percentage yield of copper oxide? 4. Why did the green powder lose mass when it changed into the black powder? What was being lost? 5. How could you prove that this was being lost?