Finding the (empirical) formula of an oxide of copper
advertisement
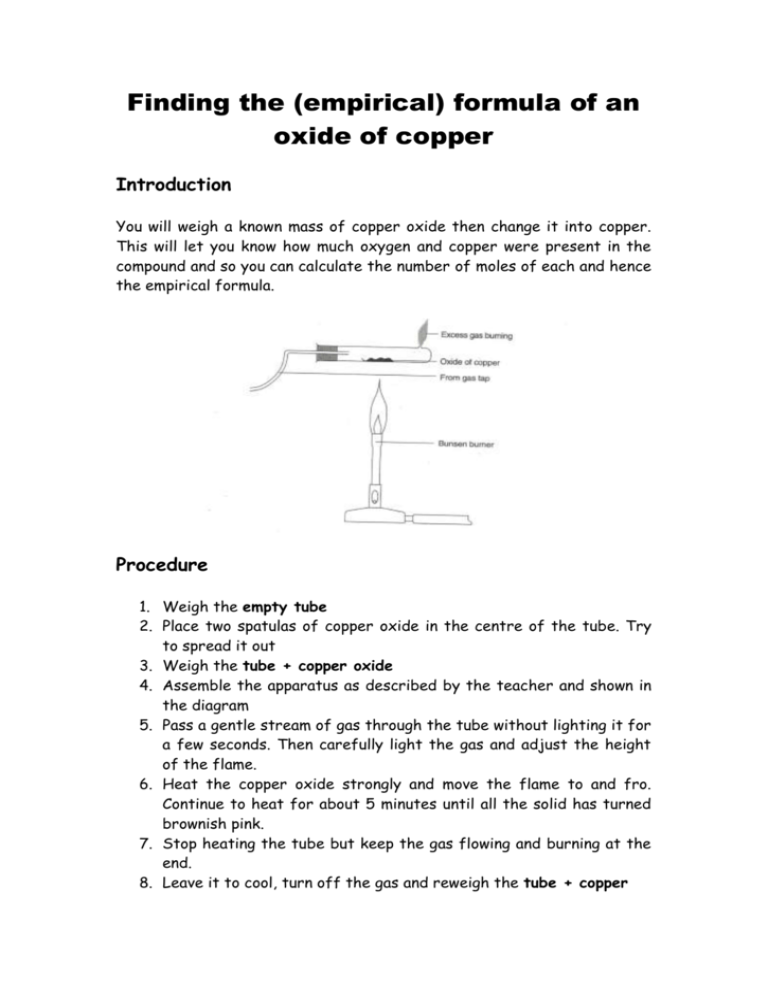
Finding the (empirical) formula of an oxide of copper Introduction You will weigh a known mass of copper oxide then change it into copper. This will let you know how much oxygen and copper were present in the compound and so you can calculate the number of moles of each and hence the empirical formula. Procedure 1. Weigh the empty tube 2. Place two spatulas of copper oxide in the centre of the tube. Try to spread it out 3. Weigh the tube + copper oxide 4. Assemble the apparatus as described by the teacher and shown in the diagram 5. Pass a gentle stream of gas through the tube without lighting it for a few seconds. Then carefully light the gas and adjust the height of the flame. 6. Heat the copper oxide strongly and move the flame to and fro. Continue to heat for about 5 minutes until all the solid has turned brownish pink. 7. Stop heating the tube but keep the gas flowing and burning at the end. 8. Leave it to cool, turn off the gas and reweigh the tube + copper Results Mass (g) Test-tube Test-tube + copper oxide Test-tube + copper Calculations 1. What was the mass of copper oxide you used? 2. What mass of copper did that form? 3. What mass of oxygen was lost? 4. How many moles of copper were formed? 5. How many moles of oxygen atoms were originally combined with this number of moles of copper? 6. What is the simplest whole number ratio of the moles? 7. What is the empirical formula of this copper oxide?









