use these fill in notes as you go thru the powerpoint
advertisement

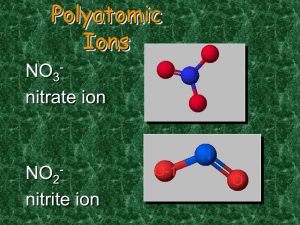
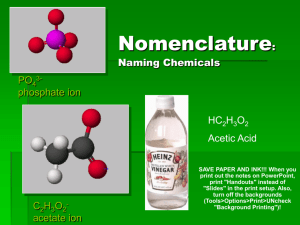
Follow along and fill in notes for Power point on Nomenclature and Bonding Name: ___________ period: __ What are the three types of bonds? _______________bonds;a transfer of electrons from one atom to another resulting in a cation and an ________. _______________bonds when valence electrons are shared between atoms in a bond ______________ bonds which are thought of as a “sea of electrons” shared by a bunch of positive ions. This helps explain their malleability and ductility. Fill-in the learning checks: Circle the correct formula for the compounds containing the following ions: 1. Na+, S22. Al3+, Cl3. Mg2+, N3- a) NaS a) AlCl3 a) MgN b) Na2S b) AlCl b) Mg2N3 c) NaS2 c) Al3Cl c) Mg3N2 Complete the names of the following binary compounds: Na3N sodium ________________ KBr potassium ________________ Al2O3 aluminum ________________ MgS _________________________ In this powerpoint it says we must memorize the ionic charge of Silver, Zinc, Cadmium and Aluminum. In our class we only have to know the charge of the Aluminum cation which is _____ There are older names for ions that don’t use roman numerals (we will always use the Roman numerals for the charges of the metals in the “valley of confusion”). In older systems the Copper (I) ion was called __________and the Copper (II) ion was called _______________ Iron (III) was called ________________ and Iron (II) was called ____________ To avoid all these problems we will use the Roman numeral system for all transition elements (the more common compound was given the “ic” name and the less common the “ous” name but what really is “common”) Complete the names of the following binary compounds with variable metal ions: FeBr2 iron (_____) bromide CuCl copper (_____) chloride SnO2 _______(_____ ) ______________ Fe2O3 ________________________ Hg2S ________________________ Circle the correct 1. aluminum nitrate a) AlNO3 b) Al(NO)3 c) Al(NO3)3 2. copper(II) nitrate a) CuNO3 b) Cu(NO3)2 c) Cu2(NO3) 3. Iron (III) hydroxide a) FeOH b) Fe3OH c) Fe(OH)3 4. Tin(IV) hydroxide a) Sn(OH)4 b) Sn(OH)2 c) Sn4(OH) Match each set with the correct name: (look at back of your periodic table for help); on the test we will only have to know the 8 polyatomic ions you are given but you should be able to determine these “ite” polyatomic ions by process of elimination. 1. Na2CO3 a) magnesium sulfite 2 . Ca(HCO3)2 a) calcium carbonate MgSO3 b) magnesium sulfate CaCO3 b) calcium phosphate MgSO4 c) sodium carbonate Ca3(PO4)2 c) calcium bicarbonate Mixed practice: Name the following: 1. Na2O ___________________ 2. CaCO3 ___________________ 3. PbS2 ___________________ 4. Sn3N2 ___________________ 5. Cu3PO4 ___________________ 6. HgF2 ___________________ Now try the other way. Write the formula: 1. Copper (II) chlorate_________________ 2. Calcium nitride ___________________ 3. Aluminum carbonate_______________ 4. Potassium bromide_________________ 5. Barium fluoride ___________________ 6. Cesium hydroxide__________________ Fill in the blanks to complete the following names of covalent compounds. CO carbon ______oxide CO2 carbon _______________ PCl3 phosphorus _______chloride CCl4 carbon ________chloride N2O _____nitrogen _____oxide CIRCLE CORRECT 1. P2O5 a) phosphorus oxide b) phosphorus pentoxide c) diphosphorus pentoxide 3. 2. Cl2O7 a) dichlorine heptoxide b) dichlorine oxide c) chlorine heptoxide write the formulas: 1. Dinitrogen monoxide_____________ 2. Potassium sulfide _____________ 3. Copper (II) nitrate ________________ 4. Dichlorine heptoxid _______________ 5. Chromium (III) sulfate ______________ 6. Iron (III) sulfite ___________________ 7. Calcium oxide ___________________ 8. Barium carbonate__________________ 9. Iodine monochloride ______________ Name ‘em • HI (aq) • HCl • H2SO4 • HNO3 • HClO3 _________________________ _________________________ _________________________ _________________________ _________________________ Cl2 a) chlorine b) dichlorine c) dichloride Name these mixed examples: 1. BaI2 _____________________ 2. P4S3 _____________________ 3. Ca(OH)2 ______________________ 4. FeCO3 ______________________ 5. Na2Cr2O7______________________ 6. I2O5 _______________________ 7. Cu(ClO3)2 ______________________ 8. CS2 _________________________ 9. B2Cl4 _________________________ try the other way (write formula) Hydrobromic acid ______________ Nitric acid ________________ Carbonic acid ________________ Phosphoric acid _______________