Mass transfer –limited bacterial growth
advertisement

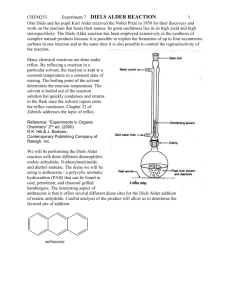
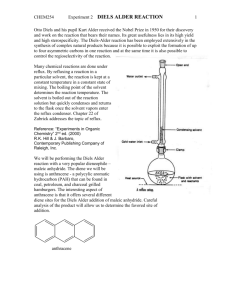
Mass transfer –limited bacterial growth on solid PAHs Jiemin Hu Department of chemical engineering, UMBC Abstract: Bacterial growth on solid polycyclic aromatic hydrocarbons (PAHs) is controlled by both the biochemical activity of microorganisms and the mass transfer of a substrate to the microorganisms. A generic mathematical concept for bioavailability is presented taking both factors into account. In this paper, Mycobacterium sp. LB501T growth is investigated with poorly soluble anthracene as sole carbon source. M. sp. LB501T is grown on various amounts of solid anthracene in batch cultures, and microbial biomass formation is compared to independently determined dissolution fluxes. The influence of substrate bioavailability on microbial growth is predicted successfully by a dynamic, flux-based approach (Best-Equation), which combines substrate dissolution from crystals into solution, substrate uptake by microorganisms from solution, and concurrent biomass formation. Introduction PAHs are environmental pollutants, which are toxic and persistent because of their hydrophobic character and low water solubility. In soil, bacteria have little chance to access the PAHs, because they are adsorbed to solid soil constituents or are dissolved in the nonaqueous phase. The desorption of sorbed substrates or the dissolution of solid substrates is considered prerequisite for biodegradation, as bacteria appear to degrade substrates only when they are dissolved in water. The rate at which microbial cells can convert substrates during biodegradation depends on two factors: (1) the rate of uptake 1 and metabolism (the intrinsic activity of the cell); (2) the rate of transfer to the cell (mass transfer). Evidently, increased microbial conversion capacities do not lead to higher biotransformation rates when mass transfer is the limiting factor. So the biotransformation is related with bioavailability, which is the ratio of the rate of mass transfer to intrinsic activity of microbial cells. When the microbial cells consume more substrate than the capacity of its environment to deliver, the overall rate is becoming mass limited. In soil, the biodegradation of PAHs is in same condition as discussed above. The overall rate is not determined by biochemical activity of microorganisms but by the physicochemical processes such as desorption, dissolution, and diffusion. Theory. Physical Modeling Scenario: Steady-State Flux Driven by Biodegradation In the experiment whose reactants are anthracene and bacteria, two steps determine anthracene concentration. First, anthracene crystals continue to dissolve till equilibrium, and have the maximum concentration Cmax. Second, the bacteria uptake the anthracene and counteract the dissolution. So the result is to reduce the bulk anthracene concentration. There is a difference between actual aqueous concentration Ct and Cmax. This difference is the driving force of continuous dissolution. When the anthracene dissolution fluxes Qd equals the bacterial degradation flux qc, the steady state is reached. Rate of Anthracene Dissolution (Qd). The film theory is appropriate to the dissolution of crystals, which assumes that the main resistance of diffusive transport is the stagnant aqueous film on the surface of crystal anthracene. According to the film theory, dissolution fluxes Qd is determined by the equation (1): Qd= k×(Cmax- Ct)= ki×Ai×(Cmax- Ct). In the equation (1), k (m3h-1) is 2 the diffusive exchange constant which is the product of the interfacial area between the crystals and aqueous phase Ai (m2) and a factor ki (m h-1) accounting for the thickness of the stagnant layer between the crystals and the liquid, which depends on the shaking velocity and the surface roughness of the crystals. Bacteria Growth on Solid Antharene. Pollutant uptake and biotransformation result in a depletion of substrate close to the bacteria, which in turn leads to a diffusion gradient between the bulk substrate and the surface of the cells. Substrate uptake and degradation are often adequately described by whole-cell Michaelis-Menten kinetics: qc=qmax×cc/(Km+cc) (2). In the equation 2, qc , the substrate flux(mol mg-1protein h-1) through the cell membrane, is the function of substrate concentration at the cell surface cc(mol m-3). Km is the cell surface concentration yielding 1/2 qmax (mol m-3), where qmax is the maximal specific degradation flux. Assuming that Cc is in equilibrium with the substrate concentration in the membrane and in the cytoplasm, the depletion rate of dissolved anthracene can be used to determine qmax and the apparent half-maximum uptake rate Km by nonlinear parameter estimation according to eq 2. At low concentrations (Cc Km), the kinetics of biodegradation is essentially first order with the ratio qmax/Km as a first-order constant. For this reason, firstorder expressions are often used to describe biodegradation of contaminants in soil, without the awareness that the first-order kinetics may be caused by mass transfer kinetics. In order to give a generic framework, this work relies on the Michaelis-Menten expression. Because anthracene is the only carbon source, part of qc is used for the synthesis of biomass and growth of the bacterial culture. Accordingly, equation 2 can be extended to 3 qcY=qmaxY×cc/(Km+cc)-b (3). In the equation 3, Y is the cell growth yield (mgprotein mol1 ) and b (h-1) reflects the substrate consumption for nongrowth processes such as endogenous metabolism and compensation for cell decay. qcY equals the specific growth rate and qmaxY the maximum growth rate max. In reality, the uptake pathway of pollutants in a soil involves a combination of various mass transfer mechanisms, e.g., dissolution from a non-aqueous phase followed by sorption retarded diffusion or the mass transfer from a flowing liquid into soil aggregates where sorption retarded diffusion takes place. Under condition of steady state, the transport flux and the degradation of the substrate equal each other, resulting in Ct Cc. Consequently, Cc and Ct can be eliminated by solving equations 1 and 3 for Cc and Ct and combining both equations to equation 4 of the flux qc as a function of Cmax only: qmax Cmax K m qmax ( Ai k i ) 1 4 Cmax qmax ( Ai K i ) 1 Y 1 1 2 qmax ( Ai k i ) 1 (Cmax K m qmax ( Ai K i ) 1 ) 2 This equation is called Best equation, which describes the control of b by the combined action of the dissolution of the crystalline substrate and microbial degradation. The biomass Xt (mg protein) at time t can be derived as: Xt2=(t2-t1) X t1 (5) Although the steady-state assumption has been used to derive the equation, it is not necessary to impose a condition as stringent as the steady state. Bioavailability Number (Bn) The important of mass transfer relative to intrinsic activity of cell is quantified in the bioavailability number Bn=k/( qmax/Km) (6), which is the ratio of the mass transfer rate to the microbial specific affinity qmax/Km. The specific affinity acts as first order constant of biodegradation when Cc Km and determines the ability of microorganisms to reduce 4 the concentration of a substrate at the cell surface. When Bn = 1, the biodegradation rate is equally controlled by the physical transport and the biochemical activity. Material and Methods Glossary In the paper, there are many terms that should be explained before further discussing the experiment and result. Ai, surface area of crystal (m2); b, microbial maintenance rate (h1 ); X, biomass (mgprotein); Cc, dissolved concentration at cell surface (mol m-3); Bn, bioavailability number; Cmax, maximal concentration in aqueous phase (mol m-3); Ct, dissolved concentration at time t during dissolution experiment (mol m-3); k, mass transfer coefficient (m3 h-1); ki, transfer constant (m h-1); ku, mass transfer coefficient per unit biomass (m3 h-1); Km, half-saturation constant of substrate uptake (mol m-3); m, maintenance coefficient (mol mgprotein h-1); qc, rate of substrate uptake (mol mgprotein-1h-1); Qd, the total quantity of anthracene dissolving per unit time from the crystals to the bulk solution (mol h-1); qmax, maximum rate of substrate uptake (mol mgprotein-1h-1); t, time (h); V, volume of solution (m3); Xmax, maximal biomass (mgprotein) maintained by substrate flux; Y, yield coefficient (mgprotein mol-1); , specific growth rate (h-1); max , maximum specific growth rate (h-1). Organisms and Culture Conditions In the experiment, the Mycobacterium sp. LB501T is the investigation object. It is an aerobic, rod-shape bacterium. It is was grown in a minimal medium containing 1 mL of a trace elements solution in the presence of various amounts of anthracene crystals with diameters of 0.2-0.5 mm as sole carbon source. The bacteria are kept in a 300 mL flask with 100mL media at 25 C on a rotatory shaker at 180 rpm. 5 Dissolution Experiments For the bacteria growth, dissolution of antharene should be prepared in the same condition as bacteria. Crystals of antharene with diameter 0.2-0.5mm are added in a flask on a rotatory shaker. At interval, samples are taken by using 1mL glass syringe, filtered by cellulose filter, and measure the concentration of aqueous anthracene by flurescene spectrophotometer. Anthracene Degradation Using Suspended Resting Cells To get the maximum concentration of aqueous anthracene, saturated solution is prepared. Excess amount of solute is added to phosphate-buffered saline (PBS; 0.1 M; pH 7.3) in a flask, and incubate on a thermostated (25 C) rotatory shaker (180 rpm) for at least 24 h. but there are some crystals left in the solution, so solution must be filtered before use by glass filter. The rate of bacteria degradation is studied at room temperature. M. sp. LB501T is pregrown on anthracene to a turbidity of OD578 = 0.3-0.6, harvested by centrifugation after residual substrate crystals were removed by filtration, washed twice, and resuspended in 100 mM PBS. The biodegradation begins after adding 1-2mL of cell suspension to anthracene solution. The anthracene is measured by reverse phase HPLC. And we use equation2 to calculate Km and qmax by the method of none linear parameter estimation. Determination of Cell Density and Protein We use spectrometer (wavelength 578nm) to calibrate the cell density after carefully shaking the sample. Cell protein can be obtained by using bovine serum albumin as protein standard, and optical density to measure the amount of protein can be gotten by the following equation: protein ( g/mL)=17.7×OD578 (R2=0.94: n=15) 6 Results and Discussion Dissolution Experiments: Dissolution mass transfer coefficients k are summarized in table1. Table 1. Kinetic Parameters of Mycobacterium sp. LB501T and Parameters Used for Modeling -1 a 0.036 ± 0.005 (n = 3b) max (h ) qmax (mol mg-1protein h-1) 7.8 × 10-6 c Km (mol m-3) 2.4 × 10-4 c Y (mgprotein/mol) 19200d b (h-1) 6.9 × 10-4 ± 1.6 × 10-4 (n = 5b) k (m3 h-1) k0.1 g/L 4.5 × 10-5 e ratio: 0.3f k0.2 g/L 1.4 × 10-4 1 k0.5 g/L -4 2.4 k1 g/L -4 4.8 × 10 3.4 k2 g/L 8.6 × 10-4 6.3 k30 g/L Cmax (mol m-3)g 3.4 × 10 -2 1.2 × 10 86 3.4 × 10-4 a Nonlinear parameter estimation (NPE) of early exponential growth in batch cultures using 30 g/L anthracene crystals.b Mean value from n independent measures with a standard deviation of ± 1 s.c NPE of n = 38 data points; R 2 = 0.93.d Value derived from suspended and adhered biomass.e k0.1 g/L was obtained by extrapolation using linear regression: k0.1 (g/L) = 4.53 × 10-4* 0.1 g/L (n = 4; R 2 = 0.94 ).f The ratio refers to kx g/L/k0.2 g/L. Dissolution transfer rates do not increase proportionally to the weight of anthracene crystals used.g Aqueous solubility at 25 C . When calculating the mass transfer coefficient, we suppose the crystal surface area is constant (Ai=0.66± 0.11 m2 g-1 (n = 3)) during the experiments. This assumption can be verified by the scanning electron microscope (SEM) analysis and BET surface area measurements. From table1, we can find that in the low concentration, the mass transfer coefficient k is direct proportional to the total crystal surface area Ai (n=5, R2=0.94), which means that k is proportional to the total amount of anthracene. But when there is a 7 large amount of anthracene (30gL-1), k is lower than expected. The reason is the clumping of crystals that might decrease the dissolution. Cmax can be found in the reference (), because the buffer solution has some impact on the concentration of anthracene (salting out), so we must consider this impact (2-5% solubility reduction). Growth of Mycobacterium sp. LB501T on Solid Anthracene According to the concentration of anthracene, we can find the linear and exponential growth in the figure 1. Figure 1 Batch growth curves of Mycobacterium sp. LB501T on 0.1. 0.2, 0.5, 1, 2, and 30 g L-1 of size fractionated anthracene crystals with a diameter of 0.2-0.5 mm. Growth is determined as optical density (OD) at 578 nm. Dissolution of 0.1, 0.2, 0.5, 1, or 2 g L-1 are reflected in mass transfer limited linear growth, whereas 30 g L-1 allow for exponential growth. SEM shows that growth can occur not only in the suspension but also on the crystal surface, where a biofilm develops (figure2). Figure 2 Cryogenic scanning electron micrograph of an anthracene surface covered by a confluent M. sp. LB501T biofilm. Direct contact of the biofilm's EPS structure (exopolysaccharides substructure) with the anthracene surface and M. sp. LB501T in close contact ( 1 m) with the anthracene surface are visible. The biofilm forms an average of 24 ± 13% of the total biomass but during growth is regularly released into the solution. 8 Figure 2 Because biomass is often measured as optical density (OD) of the bacteria suspension, so it will get a lower value of the total biomass generated due to neglecting the crystalbound biomass. But quantification of biomass on crystals shows that the ratio of suspended to crystal-bound biomass is relatively constant. In the whole period of growth, the crystal-bound biomass is 24 ± 13% (n = 10) of the total biomass, so OD measurement still can express the growth dynamics because it is proportional to cell protein( R 2=0.94, n=15). When 0.2, 0.5, 1, 2g L-1of anthracene are added, linear growth is observed after 20-40h lag phase in figure1. But it is totally different when large amount (30g L-1) is added. Exponential growth at beginning and zero growth after 11 days of incubation are found. With small amount of anthracene, the linear growth normally stops about 25 days of incubation. And the final cell number is determined by the initial concentration of solid anthracene. But this happens well before the crystals are completely consumed. The reasons are followed. Linear growth, i.e., constant biomass production, reflects continuously decreasing specific growth rates, which are controlled by a constant maximal dissolution flux that has to be shared by a continuously growing population. This proceeds until the dissolution flux to individual cells approaches the flux needed for 9 nongrowth purposes and zero growth is observed. In contrast to the final plateau cell numbers observed in exponentially growing cultures, which are likely to be due to other factors than mass transfer limitations, final cell numbers of linearly growing batches depended strongly on the initial concentration of solid anthracene This conclusion, that the final cell numbers are due to mass transfer limitations rather than other (e.g. micronutrient) deficiencies is further supported by the fact that growth resumed, when additional anthracene is given to the batch culture (Figure 3). Figure 3 Batch growth curves of Mycobacterium sp. LB501T on 0.053 (a), 0.1 (b), and 0.22 g L-1 (c) of size-fractionated anthracene crystals with a diameter of 0.2-0.5 mm: Linear growth proceeded until the dissolution flux to individual cells approached the flux needed for nongrowth purposes resulting in zero growth well before the crystals were completely used. Growth resumed after the provision of additional 0.051, 0.11, and 0.31 g L-1 of solid anthracene to cultures a, b, and c after 1020 h (cf. arrows), indicating that 10 previous final cell numbers are likely to be due to mass transfer limitations. Dashed lines represent linear curve fittings of the different growth phases. Kinetic Parameters: Km, qmax, Y, and b. By using nonlinear parameter regression, maximum rate of substrate uptake, q max, equals to 7.8*10-6mol mgprotein-1h-1 and Km equals to 2.4*10 mol m-3 which is slightly below the Cmax (Cmax = 3.4 × 10-4 mol m-3) of anthracene(these values are summarized in table1). In the process, Km and qmax are calculated based on the suspended cells, Y and b are obtained from shaken batch cultures using solid anthracene as sole carbon source. The cell growth yield Y is calculated by the following method: in the linear growth, the change of biomass ( X/ t) is basically constant because of its limitation by the maximal dissolution flux Qd. The yield Y, which is the ratio of change of biomass to the consumption of substrate, can be obtained by the following equation: Y= X / t Qd (7). The average growth obtained from linear regression by using this equation is Y=155000 mgprotein mol-1 for suspended bacteria. Because the crystal-bound biomass is 24 ± 13% of the total biomass, here we choose 24%, so for the total biomass, Y=19200 mgprotein mol-1 . This value is lower than values reported from other PAH. The reason may be explained that the biosynthesis of the exo-polysaccharides structure (EPS) in the biofilm covers the crystals (figure2). The maintenance coefficient b reflects the consumption of substrate, which does not produce growth. Thus, assuming that bacteria only grow to numbers at which the substrate flux fulfills the maintenance requirements, mass transfer limited growth will stop at a biomass Xmax when the overall substrate flux is too small to maintain further growth. With no visible growth Qd balances the flux m (mol·mg-1protein h-1) utilized for maintenance of Xmax: b=mY= Qd Y/Xmax (8). 11 Comparison of Observed and Simulated Biomass Formation We enter the obtained parameters (k, Km, qmax, Y, b; Table 1) into the Best-Equation based on lag phases of 30 h for linear and 60 h for exponential growth. We assume that diffusive transport through a stagnant layer on the surface of anthracene is the only mass transfer limited step in a well-mixed system. This assumption is verified by the figure 4 12 Figure 4 Simulated and observed growth curves of Mycobacterium sp. LB501T on 0.1, 0.2, 0.5, 1, 2, and 30 g L-1 of anthracene crystals (Figure 4a-f) based on batch maintenance rates representing total (b = 0.00069 h-1; dashed line) and crystal-bound biomass (b = 0.002 h-1; solid line). The influence of maintenance b is reflected by increasingly bent curves resulting in zero growth at biomasses directly depending on substrate fluxes. In figure4 a-e, quasi-linear growth rates with different amounts of anthracene are well predicted by the Best equation. It is the limited anthracene availability rather than microbial activity that causes quasi-linear growth at low concentration. In figure f, where large amount of anthracene is present in the system, exponential growth is simulated correctly. At low dissolution fluxes, the value of b in table1 6.9×10-4h-1 does not result in continuously bent curves and zero growth at last (figure4, dash lines). If we choose another value of b 2×10-3h-1(figure4, solid lines), which is about 3-fold higher than the calculated value. This discrepancy may be explained by the fact that (a) M. sp. LB501T grows as a confluent biofilm on anthracene and (b) no bulk anthracene concentration is detected (detection limit: <10-8 mol L-1). When b is calculated (equation 8) under the assumption that only the crystal-bound biomass, representing roughly one-fourth of the total biomass, grows and suspended cells represent shed-off, starving cells, an about four times higher maintenance rate (b = 0.0028 h-1) of the solely active biofilm cells results. This comes close to the best-fit b value. Regarding the tight coating of anthracene crystals by the biofilm (Figure 3) and the lack of dissolved anthracene in the bulk, it appears indeed likely that the substrate provision of suspended cells is at least drastically reduced as compared to biofilm cells. But based on the Best equation, there is a phenomenon that cannot be explained. In the large amount of substrate, a zero order growth occurs after exponential growth. This indicates that there are some other factors other than substrate availability, perhaps the 13 accumulation of inhibitory metabolites. A series of experiments are done to test the impact of individual parameters (k, Cmax, Km, qmax, Y, and b). The initial values are taken from table1, and each parameter varies over two orders of magnitude while keeping the others unchanged (figure5) 14 Figure 5 Evaluation of the influence of parameters k, Cmax, Km, qmax, Y, and b on growth curve calculations with 2 g L-1 crystalline anthracene. Parameters were varied individually over 2 orders of magnitude, whereas the other 5 were kept constant. Some conclusion can be gotten from figure5. We have already known that bacterial growth on solid PAHs is a mass-transfer growth. Figure5 verifies this. The biomass growth is very sensitive to k and Cmax, and insensitive to biological ability parameters (Km and qmax). As specific growth rate is proportional to Y (equation 3), any changes of Y results in proportional biomass increase (Figure 5f). The influence of b is not sensitive. Linear growth results from b=0. When b does not equal to zero, flatten curve occurs. Another way to study the mass transport is to study the parameter of bioavailability number, which expresses the substrate availability. Bn is defined in equation6. Figure 6 shows the time-dependent Bn during growth of M. sp. LB501T with and without mass transfer limitation: Figure 6 Comparison of exponential and linear growth in terms of anthracene availability as expressed by the bioavailability number (Bn): Bn < 1 and Bn 1 indicate control by mass transfer and microbial activity, respectively. When substrate concentration is small (2g/L), linear growth occurs and Bn 1, whereas high concentration (30 g L-1) at early stages of growth results in Bn 1. Bn 1 indicates exclusive control by microbial degradation, i.e., allows for exponential growth. At the 15 mid-exponential growth phase of M. sp. LB501T, however, Bn falls below unity, indicating a transition from exponential to mass transfer-limited linear growth. At the end of high concentration curve, zero growth occurs. This phenomenon cannot be explained by the Best equation. It is maybe due to the hindrance by other factors because Bn is still enough for linear growth in other cultures. Evaluation This paper is to examine the kinetics of mass transfer-limited growth of Mycobacterium sp. LB501T degrading the poorly soluble crystalline polycyclic aromatic hydrocarbon anthracene. The influence of substrate bioavailability on microbial growth is predicted successfully by a dynamic, flux-based approach (Best-Equation). But at the process of non-linear regression, some empirical data are used; these data have no theoretical base, and the Best equation cannot explain the finial zero growth of the exponential curve in the large amount of substrate. The reason of this zero growth cannot gotten, just some theoretical assumption. 16 Reference 1. Bosma, T. N. P.; Middeldorp, P. J. M.; Schraa, G.; Zehnder, A. J. B. Environ. Sci. Technol. 1997, 31, 248-252. 2. Weissenfels, W. D.; Klewer, H. J.; Langhoff, J. Appl. Microbiol. Biotechnol. 1992, 36, 689-696. 3. Bird, R. B.; Stewart, W. E.; Lightfoot, E. N. Transport Phenomena, 1st ed.; Wiley: New York, NY, 1960. 4. Koch, A. L. Adv. Microbiol. Ecol. 1990, 11, 37-69. 5. Best, J. B. J. Cell. Comp. Physiol. 1955, 46, 1-27. 6. Harms, H.; Zehnder, A. J. B. Appl. Environ. Microbiol. 1994, 60, 2736-2745. 7. Button, K. D. Microbiol. Rev. 1985, 49, 270-297. 8. Zhang, W. X.; Bouwer, E. J.; Ball, W. P. Ground Water Monit. R. 1998, 18, 126138. 17