Topic 2: Speed of Reactions
advertisement

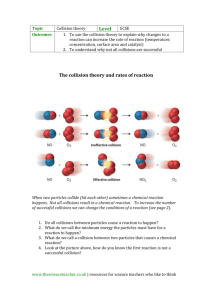
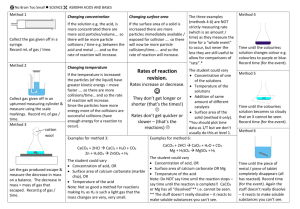
Topic 2: Speed of Reactions Changes in speeds of reaction can be explained using collision theory. Collision theory states " before any reaction can take place the reacting particles must collide with enough energy" eg H2 + O2 H2O If insufficient energy is present the reacting particles will not react. Chemical reactions can be speeded up by 1. Decreasing particle size 2. Increasing temperature 3. Increasing concentration 4. Using a catalyst 1. Decreasing particle size As the particle size decreases for a given mass the surface area increases, collisions must occur on the surface, so the greater the surface area, the more collisions that will occur, this will increase the reaction rate. e.g. iron filings react faster than iron rods. potatoes cook quicker if cut up in to small pieces. in flour mills fans extract powdered flour to prevent explosions. in mines the air is kept damp to stop coal dust catching fire. powdered metals are used in fireworks to give bright colours. 2. Increasing temperature As the temperature of the reacting particles increase, the energy of the particles increase. The number of successful collision will also increase as more collisions will have enough energy to react. e.g. foods go bad faster at room temperature than in a fridge 3. Increasing concentration When the concentration is increased the number of particles increase, this increases the number of collisions and hence the rate increases e.g. cooking food in a pressure cooker cuts down the time taken. 4. The presence of a catalyst Catalysts lower the energy needed for successful collisions resulting in the formation of products, this speeds up the reaction e.g. manganese dioxide speeds up the decomposition of hydrogen peroxide vanadium oxide in the Contact Process i.e. making sulphuric acid iron in the Haber Process i.e. making ammonia platinum in the Ostwald Process i.e. making nitric acid platinum and rhodium in catalytic converters Many catalysts such as manganese dioxide, vanadium oxide, iron and platinum contain transition elements Enzymes are biological catalysts. Enzymes are used in making cheese, yoghurt, bread, wine, beer, lager, whiskey and biological detergents. Amylase breaks down starch into glucose.The activity and efficiency of enzymes are influenced by various factors, including temperature and pH conditions. Enzymes have many medical and industrial uses, from washing powders to drug production, and as research tools in molecular biology. They can be extracted from bacteria and moulds, and genetic engineering now makes it possible to tailor an enzyme for a specific purpose.