CHAPTER 7 - Practice Exercises (ORGANIC CHEM I )
advertisement

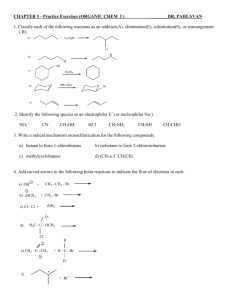
CHAPTER 7 - Practice Exercises (ORGANIC CHEM I ) ________DR. PAHLAVAN 1. Write a complete reaction for the followings. Please show the stereochemistry of the product and explain why a mixture is formed (if any). a) b) c) d) e) Dehydration of 3-methyl-3-hexanol in aqueous sulfuric acid Treatment of 2-bromo-2-methylbutane with KOH in ethanol Treatment of 1,2-dimethylcyclohexene with Cl2 Addition of HCl to 1,2-dimethylcyclohexene Reaction of cyclopentene with NBS and water in DMSO 2. Predict the products of the reactions of followings compounds with given reagents. Please indicate regiochemistry when relevant. III) II) I) a) H2 /Pt b) Br2 c) HBr g) Hg(oAc)2 , H2O / NaBH4 d) OsO4/NaHSO3 f) KMnO4 /H3O i) BH3,THF / H2O2, OH h) O3 / Zn, H3O k) CH2I2 / Zn(Cu) j) CHCl3 / KOH e) Cl2 /H2O l) Cl2 / CH2Cl2 3. Describe how to distinguish between the members of the following pairs of compounds by a simple chemical test. For the pair, tell what test to perform, what you expect to observe, and write an equation for each positive test. For example, to distinguish between cyclohexane and 1-hexene, you might consider the reaction of each with Br2 in CCl4 or reaction with KMnO4 in basic solution. a) cyclohexane and 1-hexene b) 1-hexene and 2-chlorohexane 4. How would you carry out the following transformations? Indicate the reagents you would use in each case? OH a) ? ? b) OH ? CH3 c) d) Cl OH C Cl e) ? H O O 1 ? CH3 5. Suggest structures for alkenes that give the following reaction products. There may be more than one answer for same cases. CH3 H2 / Pt, C CH3 ? a) Cl HCl ? b) CH3 1) Hg (OAc)2, H2O ? c) 2) NaBH4 OH Br Br2 d) ? Br O e) ? O3 / Zn , H3O O H - C - CH2 - CH2 - CH2 - CH2 - C - CH3 6. Road map problem- What are the structures of compounds A, B, C, and D? C8H16 (C) 4 H2 reduce aromatic ring Compound A (C8H8) KMnO4 CO2 + Carboxylic acid B ( C7H6O2) 1 molar equivalent H2 /Pd Compound (D) 7. A compound has a formula C10H16. On catalytic hydrogenation over palladium, it reacts with only 1 mole equivalent of H2. Compound A also undergoes reaction with ozone, followed by zinc treatment, to yield a symmetrical diketone B (C10H16O2). Identify the structures for compounds A and B. 2 8. Show how to convert cyclopentene into these compounds. Please show all the steps clearly. Br a) OH b) Br d) OH c) OH O e) f) Br O g) I C - (CH2)3 - C H H 9. Propose a mechanism, using curved arrows to show electron movement for each step. a) hydrobromination of 1-methylcyclohexene b) acid catalyzed addition of 2-methylpropene with CH3OH in presence of H2SO4 c) bromination of 1-methylcyclohexene d) oxymercuration hydration of 1-methylcyclohexene in sodium borohydride e) bromohydration of 2-methypropene 10. Show by a series of reactions how could you prepare the following compounds from the indicated starting compound. Be sure to clearly indicate the reagents used in each step. OH a) Cl OH O O b) H CH3 c) H C - (CH2)4 - C CH3 Br Br d) CH3 -CH2 - CH2 - CH2Br CH3 -CH2 - CHBr - CH3 3 11. Draw structural formulas for the products of the following reactions. Predict which is the major product. CH3 H2O / H2SO4 a) CH3 - C = CH - CH3 Br2 / CCl4 b) CH3 1) BH3, THF c) 2) H2O2 , OH NBS / light d) ( allylic bromination) NBS e) f) H2O , DMSO (halohaydration) 1) O3 CH3 - C = CH - CH2 - CH3 2) Zn, H3O CH3 Br g) CH3 - CH - CH - CH3 KOH heat CH3 Br2 /H2O h) i) 1) Hg(OAc)2 , H2O 2) NaBH4 j) KMnO4 4