File - Garbally Chemistry
advertisement

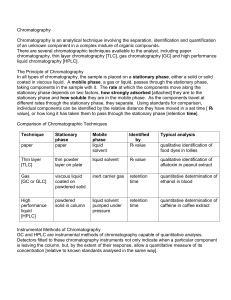
Chromotography and Instrumentation Seperation technique in which a mobile phase (water, ethanol) carrying a mixture moves in contact with a selectively absorbent (paper, alumina, silica gel) stationary phase. Paper Chromatography. Method a) A small amount of the mixture to be separated is placed on a strip of chromatography paper. b) The end of the paper is placed in a suitable solvent. c) The solvent soaks up through the paper and dissolves the sample of material being analysed. Each of the components of the mixture dissolves to a certain extent. The least soluble come out of solution and appear as a spot on the paper. d) Thus the original sample will be split into its various component sat different intervals along the paper. Note: Avoid handling the paper as oils from the hands can affect the result. Figure 1 The separation process Thin Layer Chromotography. Stationary Phase Thin layer of aluminium oxide or silica gel on a glass plate or aluminium foil. Method A spot of the mixture been analysed is placed near the bottom of the plate. When the spot is dried the TLC plate is placed standing in a suitable solvent. TLC is more efficient than paper in that the particles in the stationary phase are smaller and this gives better separation of components in the mixture. Uses Pharmaceutical industry - to determine the purity of drugs. Forensic science-To seperate the colours in dyes extracted from a crime scene. Analysis of drugs like cannabis Figure 3 Thin Layer Chromatography (TLC) Column Chromatography Stationary Phase-made into slurry using an appropriate solvent. The mixture to be separated is dissolved in a solvent and added to the top of the column. The solvent is allowed to pass through the column and is kept moist. If the column is long a series of coloured bands will appear along the column. This series of bands is called column chromatography. The procedure of passing a solvent through the column is called elution. The general term for the solvent is the eluent. Figure 2 Column chromatography Gas Chromatography. Mobile Phase=Gas Stationary Phase= a high Boiling Point liquid spread on solid particles(alumina)that are packed into a long coiled tube called the column. This coiled tube of small diameter and several meters in length is kept inside a temperature controlled oven. Method a) The sample is injected, vaporised, and carried through the tube using an inert gas like Helium, Nitrogen, or Argon. b) The various components in the mixture separate as they flow through the column. c) As each component leaves the column, it passes a detector which records a signal. Uses Level of alcohol in blood or Urine. Drug test athletes . GC-MS. Gas Chromatography + Mass Spectrometry. Each individual component analysed. Analyse performance of drugs in body Detect gases from waste dumps and organic pollutants in water. High Performance Liquid Chromatography. (HPLC) The substance to be analysed is added to a liquid solvent and passed over a solid. Better separation if the particles in the stationary phase are very small. A pump is used, therefore the column need not be as long as in Gas Chromotography.Another advantage is that HPLC is carried out at a lower temperature and can analyse compounds that would decompose at a higher temperature. Used Analysis of foods- presence of growth promoters - presence of additives - Vitamins. Figure 4 Schematic diagram of an HPLC instrument Mass Spectrometry (Francis William Aston) - built to measure the masses of atoms. Detect the presence of Isotopes. A Mass Spectrometer is used to i. Identify the presence of Isotopes ii. Measure the relative abundance of isotopes iii. Measure relative atomic masses and relative molecular masses iv. Identify unknown compounds. How a spectrometer works. 1. Vaporisation 2. Ionisation 3. Acceleration 4. Separation 5. Detection Uses of a Mass Spectroscope Used in conjunction with Gas Chromatography to detect banned drugs in athletes. Test for drugs taken by drug addicts Analyse the gases in the tail of a comet. UV spectrometry Used to detect the presence of certain functional groups in molecules . Also used to detect or measure the concentration of organic compounds in solution. Measure the amount of drug metabolite reaching a certain part of then body. Figure 4 A modern GC-MS instrument at Tallaght R.T.C