Electronic supplementary information
advertisement

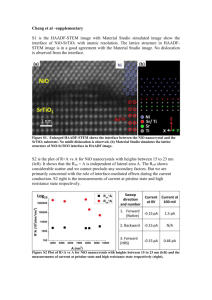
Supplementary Material for Journal of Porous Materials Supporting Information Highly ordered crystalline mesoporous metal oxides for hydrogen peroxide decomposition Mingshi Jin Jung-Nam Park* Jeong Kuk Shon Zhenghua Li Eunok Lee Ji Man Kim* Department of Chemistry, BK21 School of Chemical Materials Science and Department of Energy Science, Sungkyunkwan University, Suwon, 440-746, Republic of Korea *Corresponding authors. E-mail address: pjungnam@gmail.com (Jung-Nam Park); jimankim@skku.edu (Ji Man Kim) 1. Preparation of meso-MOx by nano-replication method The typical syntheses of mesoporous KIT-6 [1], CeO2, Co3O4, Cr2O3, Mn2O3 [2], NiO, RuO2, SnO2 [3] and TiO2 are as follows. 1.1 Synthesis of Ia3d mesoporous silica KIT-6 and surface modified HP-KIT-6 In a typical synthesis, mesoporous silica template, KIT-6, was synthesized following methods reported elsewhere [1]. A triblock copolymer (Pluronic P123, EO20PO70EO20, Aldirch) and tetraethylorthosilicate (TEOS, Aldrich) were utilized as the structure-directing agent and framework source, respectively. The template was removed by extraction in an ethanol-HCl mixture (addition of 1 g of product in the 1 mixture of 20 ml ethanol and 2 ml concentrated HCl) for 1 h, followed by calcination at 550 °C for 3 h. For modication of KIT-6 surface, after calcination, the silica surface of KIT-6 was modified with methyl groups by refluxing a mixture containing 0.6 g hexamethyldisilazane (HMDS, 99%, Fluka), 150 mL of n-hexane and 3.0 g of calcined KIT-6. The material thus obtained is denoted as surface modified HP-KIT-6 (hydrophobic KIT-6). 1.2 Synthesis of meso-Co3O4, Cr2O3, Mn2O3, NiO and SnO2 The KIT-6 material was used as a template for the synthesis of meso-MOx replica [2]. For synthesis of meso-Mn2O3, Manganese (III) nitratehexahydrate (Mn(NO3)2·6H2O, Aldrich) was used as meso-Mn2O3 precursor. Typically, 5.0 g of the calcined KIT-6 template was heated at 100 oC for 1 h. The pre-heated silica template was poured into a polypropylene bottle containing 4.63 g of Mn(NO3)2·6H2O (m.p. 37 oC) that was melted to liquid phase at 80 oC. The bottle containing the mixture was closed and shaken vigorously to mix the Mn(NO3)2·6H2O and KIT-6 template. Subsequently, the bottle was put in an oven at 80 oC overnight for the spontaneous infiltration of manganese precursor within the mesopores of silica template. The composite materials then were heated to 450 oC under ambient atmosphere for 3 h. The silica template was removed by treating the composite material with 2 M NaOH solution three times. Finally, the meso-Mn2O3 material thus obtained was washed with distilled water and acetone several times and dried at 80 oC. Elemental analysis indicated that the amount of residual silica template was below than 0.1 wt%. For the synthesis of Co3O4, Cr2O3, NiO, SnO2, and TiO2 the precursors (Aldrich); Co(NO3)2·6H2O (99%), Cr(NO3)3·9H2O (99%), Ni(NO3)2·4H2O (98%), RuCl3·3H2O (99%), SnCl2·2H2O (99%), Ti(OCH(CH3)2)4 (99%) were used respectively. The synthesis of meso-CeO2, Co3O4, Cr2O3, Mn2O3 NiO, SnO2, and TiO2 is identical to that of meso-Mn2O3. 1.3 Synthesis of meso-RuO2 using surface modifed KIT-6 2 HP-KIT-6 (hydrophobic KIT-6) was used to synthesize the meso-RuO2. The solution (containing 1.97 g RuCl3 (99%, Aldrich), 1.5 g EtOH and 1.5 g distilled water) was impregnated into 5.0 g of the modified KIT-6 template by an incipient wetness method. After drying at 80 oC for 24 h, the composite was heated to 300 oC under nitrogen flow for 24 h. Subsequently, the silica template was completely removed by treating the composite material with 3.0 M NaOH solution three times at 0 oC. Finally, the meso-RuO2 material was washed with distilled water and acetone several times and dried in a vacuum oven at room temperature for 12 h. 2. Preparation of bulk-MOx by conventional precipitation method 2.1 Synthesis of bulk-CeO2, Co3O4, Cr2O3, Mn2O3, NiO and SnO2 The typical synthesis of bulk-CeO2 [4], Co3O4 [5], Cr2O3 [6], Mn2O3 [7], NiO [8] and SnO2 [9] are as follows. For the synthesis of CeO2, 30 mL of 1.0 M of NH4OH was added drop by drop in to 100 mL of 0.1 M aqueous solution of Ce(NO3)3·6H2O, leading to the formation of a precipitation. During the precipitation, the formed suspension was strongly mixed. A precipitate was then collected by vacuum filtration, washed with distilled water several times, dried at room temperature for 48 h, and finally calcined at 500 oC under air environment for 3 h. The Co3O4, Cr2O3, Mn2O3, NiO, RuO2 and SnO2 were also synthesized by the precipitation method with identical procedure using corresponding metal precursor with meso-MOx. 2.2 Synthesis of bulk-RuO2 For the preparation of RuO2 [10], 60 mL of 1.0 M of NH4OH was added drop by drop in 200 mL of 0.02 M aqueous solution of RuCl3·3H2O (99%), leading to the formation of a precipitation up to pH 5.1. During the precipitation, the formed suspension was strongly mixed. And then, 30% H2O2 solution was added drop by drop in the mixture and stirred for 4 h. A black precipitate was then collected by vacuum filtration, washed with distilled water. Wet precipitates were redispersed into 40 ml of distilled water using an ultrasound bath, then autoclaved at 160 oC for 2 h. 3 The precipitates was then collected by vacuum filtration, washed with distilled water several times, dried at room temperature for 48 h, and finally calcined at 500 oC under air environment for 3 h. References [1] J.K. Shon, S.S. Kong, J.M. Kim, C.H. Ko, M .Jin, Y.Y. Lee, S.H. Hwang, J.A. Yoon, J.N. Kim, Chem.Commun. 650 (2009) [2] J.-N. Park, J. Shon, M. Jin, S. Kong, K. Moon, G. Park, J.-H. Boo, J. Kim, Reac. Kinet. Mecha. Catal. 103,87 (2011) [3] J.K. Shon, S.S. Kong, Y.S. Kim, J.-H. Lee, W.K. Park, S.C. Park, J.M. Kim, Microp. Mesop. Mater. 120, 441 (2009) [4] M.J. Godinho, R.F. Goncalves, L.P.S. Santos, J.A. Varela, E. Longo, E.R. Leite, Mater. Lett. 61,1904 (2007) [5] C.-B. Wang, C.-C. Lee, J.-L. Bi, J.-Y. Siang, J.-Y. Liu, C.-T. Yeh, Catal. Today 146,76 (2009) [6] Z. Pei, Y. Zhang, Mater. Lett. 62,504 (2008) [7] S. Ordόṅez, J.R. Paredes, F.V. Díez, Appl. Catal. A: Gen 341,174 (2008) [8] X. Deng, Z. Chen, Mater. Lett. 58, 276 (2004) [9] L. Xi, D. Qian, X. Tang, C. Chen, Mater. Chem. Phys. 108,232 (2008) [10] S. Music, S. Popovic, M. Maljkovic, A. Saric, Mater. Lett. 58,1431 (2004) 4 Table S1. The used amounts of each precursor for synthesis of meso-MOx catalysts Sample KIT-6 meso-CeO2 meso-Co3O4, meso-Cr2O3 meso-Mn2O3 meso-NiO meso-RuO2 meso-SnO2 meso-TiO2 Precursor type Ce(NO3)3∙6H2O Co(NO3)2∙6H2O Cr(NO3)3·9H2O Mn(NO3)2∙6H2O Ni(NO3)2∙6H2O RuCl3∙3H2O SnCl2∙6H2O Ti(OCH(CH3)2)4 Amount (g) 5 3.19 6.16 2.37 4.50 4.86 1.73 5.09 6.72 5 Table S2. The related standard reduction potentials of metal ion, metal oxide and metal hydroxide species of studied catalysts Metal ion species Metal oxide and metal hydroxide species Catalyst Reaction E0 (V)a E0 (V)a CeO2 Ce4+ + e ⇔ Ce3+ 1.72 Ce(OH)3+ + H+ + e ⇔ Ce3+ + H2O 1.715 Co3O4 Co3+ + e ⇔ Co2+ 1.92 Co(OH)3 + e ⇔ Co(OH)2 + OH- 0.17 Cr(V) + e ⇔ Cr(IV) 1.34 Cr2O72- + 14H+ + 6e ⇔ 2Cr3+ + 7H2O 1.232 Mn2O3 + 6H+ + e ⇔ 2Mn2+ + 3H2O 1.485 MnO4- + 4H+ + 3e ⇔ MnO2 + 2H2O 1.679 Mn(OH)3 + e ⇔ Mn(OH)2 + OH- 0.15 Ni(OH)2 + 2e ⇔ Ni + 2OH- -0.72 RuO2 + 4H+ +2e ⇔ Ru2+ 2H2O 1.120 SnO2 + 4H+ + 2e- ⇔ Sn2+ + 2H2O -0.094 TiO2 + 4H+ + 2e ⇔ Ti2+ + 2H2O -0.502 Cr2O3 Cr + e ⇔ Cr 3+ Mn2O3 NiO 2+ Mn3+ + 3e ⇔ Mn2+ -0.407 1.541 Ni2+ + 2e ⇔ Ni -0.257 Ru3+ + e ⇔ Ru2+ 0.248 Ru + 2e ⇔ Ru 0.455 Sn2+ + 2e ⇔ Sn -0.137 Sn4+ + 2e ⇔ Sn2+ 0.151 RuO2 2+ SnO2 Ti3+ + e ⇔ Ti2+ -0.9 TiO2 Ti + 3e ⇔ Ti 3+ a Reaction -1.37 : the values of standard reduction potentials 6 Fig. S1. N2 adsorption-desorption isotherms of bulk-MOx. a) CeO2, b) Co3O4, c) Cr2O3, d) Mn2O3, e) NiO, f) RuO2, g) SnO2, and h) TiO2 7 Fig. S2. O2-TPO patterns of (a) meso-MOx, and (b) bulk-MOx. a) CeO2, b) Co3O4, c) Cr2O3, d) Mn2O3, e) NiO, f) RuO2, g) SnO2 and h) TiO2 8 Fig. S3. H2-TPR patterns of (a) meso-MOx, and (b) bulk-MOx. a) CeO2, b) Co3O4, c) Cr2O3, d) Mn2O3, e) NiO, f) RuO2, g) SnO2 and h) TiO2 9