Lesson4_Ionic bonding individual worksheet
advertisement

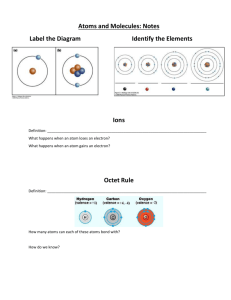
Regents Chemistry Nomen:_______________________ Lesson 4: DWBAT explain how metals and nonmetals combine to form ionic bonds Model 1: A Lewis dot diagram for an atom shows the symbol for the element surrounded by dots representing the atom’s valence electrons. By convention, the dots are arranged in this order… Using a single color of fruit loops (e.g., all green) and its symbol, make a Lewis dot diagram for sodium on the sheet provided. Using a different color of fruit loops, make a Lewis dot diagram for chlorine. Q1. Copy the Lewis dot diagrams for Na and Cl below. Use colored pencils. Q2. Write the ground state Bohr notation electron configuration for neutral atoms of: sodium: chlorine: Q3. Which noble gas is closest to Na on the periodic table? ______ For sodium to have a noble gas configuration by gaining e- how many e- would it have to gain? _________ For sodium to have a noble gas configuration by losing e- how many e- would it have to lose? _________ Recall that metals like sodium have low 1st ionization energies. Which is more likely to happen? Q4. For chlorine to have a noble gas configuration by gaining e- how many e- would it have to gain? _________ For chlorine to have a noble gas configuration by losing e- how many e- would it have to lose? _________ Recall that non-metals like chlorine have high 1st ionization energies. Which is more likely to happen? Model 2: Ionic bonds form when an atom of one element transfers electrons to an atom of another element. The atom that loses electrons to have a noble gas configuration gives one or more electrons to the atom that gains electrons to have a noble gas configuration. The atoms are no longer neutral; they are now ions, because they have gained or lost electrons. One ion will be positively charged, and the other is negatively charged. The opposite charges of the ions attract them to one another forming what is called an ionic bond. Q5. In the case of Na and Cl, which atom will transfer electrons to which atom? Show this electron transfer on your fruit loop diagram, and then copy the final result (after the transfer) using colored pencils. Note: A full valence shell should have 8 dots, while an empty valence shell should have no dots. Q6. What are the charges of the sodium and chlorine ions? Add these charges to your diagram above at the upper righthand corner of the symbol for each ion. Stop here and show your instructor your work. Using 2 different colors of fruit loops, make Lewis dot diagrams for neutral beryllium and sulfur atoms on the sheet provided. Q7. Copy the Lewis dot diagrams for Be and S below in color: 1 Regents Chemistry Nomen:_______________________ Q8. Write the ground state Bohr notation electron configuration for neutral atoms of: beryllium: sulfur: Q9. Which noble gas is closest to Be on the periodic table? ______ For beryllium to have a noble gas configuration by gaining e- how many e- would it have to gain? _________ For beryllium to have a noble gas configuration by by losing e- how many e- would it have to lose? _________ Which is more likely to happen? Q10. For sulfur to have a noble gas configuration by gaining e- how many e- would it have to gain? _________ For sulfur to have a noble gas configuration by losing e- how many e- would it have to lose? _________ Which is more likely to happen? Q11. In the case of Be and S, which atom will transfers electrons to which atom? Show this electron transfer on your fruit loop diagram, and then copy the result below in color. Include the charges of each ion. Stop here and show your instructor. Using 2 different colors of fruit loops, make a Lewis dot diagrams for neutral calcium and fluorine atoms on the sheet provided. Q12. Copy the Lewis dot diagrams for Ca and F below: Q13. Which element is more likely to lose electrons to have a noble gas configuration, and which element is more likely to gain electrons? Model 3: Compounds in which ions are bonded together via ionic bonds are called ionic compounds. In an ionic compound, each ion must have a full valence shell. The total number of electrons gained by one element must be equal to the number of electrons lost by the other element. Sometimes, the ions do combine in ratios that are NOT 1:1 Q14. Using your fruit loops Lewis dot diagrams, show how calcium can form an ionic compound with fluorine. Note: You may need more than one Ca atom and/or more than one F atom. Make additional Lewis dot diagrams, as needed. Show your instructor your work. Q15. Copy the Lewis dot diagrams for the ionic compound made of Ca and F below. Make sure to include the charges on the ions. 2 Regents Chemistry Nomen:_______________________ Summary Questions S1. List all of the metallic elements used in this activity. S2. When the metals formed ions they (circle one) gained/lost electrons to form (circle one) negative/positive ions. S3. List all of the nonmetallic elements used in this activity. S4. When the nonmetals formed ions they (circle one) gained/lost electrons to form (circle one) negative/positive ions. S5. When Na becomes an ion, what is its Bohr electron configuration? Which noble gas has the same configuration? S6. When Cl becomes an ion, what is its Bohr electron configuration? Which noble gas has the same configuration? S7. Compare the charges of the ions in this activity to the oxidation states listed for those elements on the periodic table. S8. When Be forms a bond with S, what is the net charge (overall charge) on the compound formed? S9. Why do Ca and F combine in a ratio that is not 1:1? S10. Create a fruit loop Lewis dot diagram for the ionic compound between Sr and N. You will need more than one Sr and more than one N. Copy the diagram in the space below. Critical Thinking Questions Model 4: Transition metals (Groups 3-12) have valence electrons in 2 different shells, and can form ions with different charges depending on how which electrons are lost. To determine the charge of an ion of a transition metal, we will look at its oxidation states on the periodic table and NOT its Bohr electron configuration. C1. Iron is listed as having 2+ and 3+ oxidation states. What happened to its electrons if Fe forms an ion with a charge of 2+? What about 3+? C2. Assume you have an ion of iron that has lost 3 valence electrons. Using Lewis dot diagrams (fruit loops optional), show how this atom of iron would bond with chlorine. How would it bond with sulfur? 3