Supplementary data
advertisement

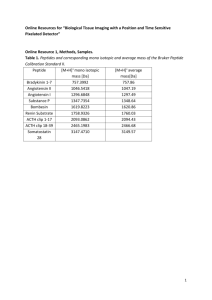
Supplementary data Chaperone nanobodies protect gelsolin against MT1-MMP degradation and alleviate amyloid burden in the gelsolin amyloidosis mouse model Authors Wouter Van Overbeke1, Adriaan Verhelle2, Inge Everaert3, Olivier Zwaenepoel1, Joël Vandekerckhove1, Claude Cuvelier3, Wim Derave2, Jan Gettemans1,* Materials and methods Antibodies and reagents Penta-His6 horse radish peroxidase (HRP) was obtained from Qiagen (Venlo, The Netherlands). Polyclonal anti-FAF antibody (1:2000 in Western blot), raised against GSTtagged 8 kDa amyloidogenic peptide, was kindly provided by dr. Jeffery Kelly (Scripps Institute, CA, USA). Polyclonal anti-CapG antibody [1] and polyclonal anti-gelsolin antibody [2] were diluted 1:2000 for Western blot. Anti-mouse/rabbit IgG HRP conjugate was purchased from VWR (Leuven, Belgium), monoclonal anti-laminin antibody (1:500) was obtained from Abcam (Cambridge, UK). Alexa Fluor 594 goat anti-rabbit, 488 goat antimouse and 594 goat anti-rat antibody IgG (H+L) were purchased from Life Technologies (Merelbeke, Belgium). The catalytic domain of MT1-MMP was obtained from VWR (Leuven, Belgium). Collagen type I was purchased from Corning (Tewksbury, MA, USA). Mouse serum albumin, recombinant human furin and polyclonal anti-V5 antibody (1:2000 in Western blot) were purchased from Sigma-Aldrich (Diegem, Belgium). Monoclonal anti-V5 antibody (1:5000 in ELISA or 1:500 in immunohistochemistry) was purchased from Life Technologies (Merelbeke, Belgium). NH2-CVHVSEEGTEPEA-COOH peptide (plasma gelsolin 231V- 242A) was synthesized on an Applied Biosystems automated 431A peptide synthesizer. An N-terminal cysteine residue was added for subsequent coupling to keyhole limpet haemocyanin and the synthesis was performed using Fmoc chemistry according to the manufacturer’s instructions. The peptide was cleaved from the resin with trifluoroacetic acid and desalted in water using a Sephadex G-25 gelfiltration column (30 × 2.6 cm). The peptide was further purified by preparative C4 reverse-phase HPLC and subsequently lyophilized. Next, the peptide was coupled to keyhole limpet haemocyanin and polyclonal anti-rabbit antibodies were raised against this peptide (Centre d’Economie Rurale, Laboratoire d’hormonologie, Marloie, Belgium). The rabbit antiserum is referred to as anti-8 kDa antiserum and was diluted 1:2000 (Western blot) or 1:500 (immunohistochemistry). The 8 kDa human gelsolin FAF peptide, 173 ATEVPVSWESFNNGNCFILDLGNNIHQWCGSNSNRYERLKATQVSKGIRDNERSG RARVHVSEEGTEPEAM243, used for raising nanobodies (see further), was chemically synthesized by Caslo (Denmark). The peptide was N-terminally biotinylated, followed by a C6 carbon spacer and the C-terminus was amidated. Generation of FAF gelsolin-specific nanobodies Nanobodies were obtained in collaboration with VIB Nanobody Service Facility. A dromedary was injected subcutaneously on days 0, 7, 14, 21, 28 and 35 with 85 g biotinylated human gelsolin FAF peptide combined with 150 µg neutralite avidin per injection. The same animal was immunized with 200 µg GST-tagged recombinant domain 2 (132G – 286S) of human D187N gelsolin per injection (G2D187N). On day 39, anticoagulated blood was collected for the preparation of lymphocytes. From these lymphocytes, total RNA was extracted and a cDNA library was made using oligo-dT primers. The VHH cDNA fragments were cloned in the phagemid pHEN4 vector in order to perform phage panning using the 8 kDa peptide or G2D187N. Enrichment for antigen-specific phages was assessed after each round of panning by comparing the number of phagemid particles eluted from antigen-coated wells with the number of phagemid particles eluted from wells blocked with blocking solution. For both antigens, hundreds of individual colonies were randomly selected and analyzed by ELISA for the presence of antigen-specific VHHs in their periplasmic extracts. Out of these colonies, 5 scored positive for the 8 kDa peptide and 15 scored positive for gelsolin domain 2. Nucleotide sequencing revealed that the 5 colonies positive for the 8 kDa peptide represented 1 nanobody (originally named FAF Nb1). The 15 positive colonies for G2D187N represented 3 different nanobodies but belonging to the same group. One of these was identical to FAF Nb1. Hence, 3 distinct nanobodies were identified and will be referred to in this work as FAF Nb1-3. Supplementary Figures Figure S1. Establishing FAF Nb1-3 specificity The specificity of FAF Nb1-3 was tested in a co-immunoprecipitation assay using E. coli lysates expressing recombinant C68, PG or PG*. The crude lysate is shown (20 µg) and 1 mg lysate was incubated with 5 µg nanobody (left lane). FAF Nb1-3 (shown in a, b and c, respectively) bind C68, PG and PG*, albeit to varying degree (arrows). A bacterial lysate containing GST-CapG was used as negative control and none of the FAF Nbs coimmunoprecipitated GST-CapG (boxes). (d) Non-specific interaction of recombinant proteins was tested by incubating the baterial lysate with anti-V5 agarose. (*anti-V5 Ab light chain; **anti-V5 Ab heavy chain). Gels were stained with Coomassie blue. Figure S2. FAF nanobodies do not prevent gelatin degradation by MT1-MMP Nanobody substrate specificity was checked in an in vitro degradation assay in which gelatin (heat denatured collagen type I) was degraded by MT1-MMP in the absence or presence of FAF Nb1 and FAF Nb2-MSA21. (a) In vitro breakdown of FAF nanobodies was investigated to discriminate nanobody breakdown from gelatin proteolysis. Lanes 1-2: 5 µg FAF Nb1 and FAF Nb2-MSA21, respectively, without MT1-MMP. Lanes 3-4: both nanobodies incubated with MT1-MMP for 2 hours (37°C), without gelatin. (b) Uncleaved gelatin presents as two doublets in Coomassie stained denaturing gels (top, lane 1). Following MT1-MMP proteolysis, cleavage products are detectable in the lower molecular weight range (bottom, lane 2). FAF Nb1 and FAF Nb2-MSA21 do not prevent this proteolysis (bottom, lanes 3 and 4, respectively). Addition of TIMP-2 (a natural MMP inhibitor) results in protection of gelatin from cleavage (bottom, lane 5, TIMP-2 is indicated by the arrow). Figure S3. MSA21 linked FAF Nb1-3 retain the ability to bind C68 in ELISA A dilution series of bispecific FAF Nb was used, starting from 1 µg (= 1) up to 10 -5 µg. MSA21 linked FAF Nb1-3 (shown in a, b and c, respectively) displayed concentration dependent interaction (measured at 450 nm) with C68 (black bars). Non-specific interaction was tested with GST-CapG coated wells, acting as a negative control (white bars). Signals are represented relative to the highest value, normalized to 1. Figure S4. Specificity of the anti-8 kDa antiserum (a) A bacterial lysate of E. coli cells expressing C68, PG, PG* or GST-CapG (negative control) was fractionated by SDS-PAGE, blotted and analysed using the anti-8 kDa antiserum as primary antibody. Left lane: the antiserum did not react to a significant degree with GSTCapG or bacterial proteins. The antiserum recognized C68, PG and PG* (arrows). Degradation products were detected in the C68 bacterial lysate. (b) The same procedure was repeated for the E. coli lysate containing the 8 kDa peptide; monomeric 8 kDa peptide and oligomers are visualized. Figure S5. AST/ALT levels are not significantly altered in nanobody treated gelsolin amyloidosis mice. Plasma levels of AST (aspartate transaminase) and ALT (alanine transaminase) were measured in plasma of the gelsolin amyloidosis mice post-nanobody treatment. Measurements were performed in the FAF Nb2-MSA21 group (white bars) since this nanobody has the longest half-life (± 1 week) and data were compared to the PBS group (black bars). No significant differences were measured for both AST and ALT levels. Figure S6. FAF Nb2-MSA21 injection has a benefical effect on contraction speed during fatigue in EDL and force-frequency relationship in soleus (a,b) Repeated in vitro muscle contractions in FAF Nb1 (grey circles) and FAF Nb2-MSA21 (white circles) injected gelsolin amyloidosis mice compared to PBS controls (black circles). Injections with FAF Nb2-MSA21, but not with FAF Nb1, resulted in a higher contraction speed in the beginning of fatigue in EDL (a) but not in soleus (b), * p < 0.05 FAF Nb2MSA21 vs. PBS. (c,d) In vitro muscle contractions at different frequencies in FAF Nb1 (grey circles) and FAF Nb2-MSA21 (white circles) inject gelsolin amyloidosis mice compared to PBS controls (black circles). (c) No significant between-group differences were detected in EDL. (d) In soleus, relative forces (expressed relative to maximum force) are higher at 75 and 100Hz in FAF Nb2-MSA21 injected mice compared to PBS (* p < 0.05) and higher at 1, 35 and 50Hz in FAF Nb2-MSA21 injected mice compared to FAF Nb1 ($ p < 0.05) (two sided unpaired t-test). References 1. Van Impe, K, Bethuyne, J, Cool, S, Impens, F, Ruano-Gallego, D, De Wever, O, et al. (2013). A nanobody targeting the F-actin capping protein CapG restrains breast cancer metastasis. Breast cancer research : BCR 15: R116. 2. Van den Abbeele, A, De Corte, V, Van Impe, K, Bruyneel, E, Boucherie, C, Bracke, M, et al. (2007). Downregulation of gelsolin family proteins counteracts cancer cell invasion in vitro. Cancer letters 255: 57-70. Table S1a. Isothermal titration calorimetry (ITC) parameters for FAF Nb1-3 a FAF Nb1 FAF Nb2 FAF Nb3 Na 1.1 ± 9.7 x 10-2 1.1 ± 5.5 x 10-2 1.1 ± 4.5 x 10-2 Kd (M) 3.8 x 10-7 ± 1.2 x 10-8 8.4 x 10-7 ± 4.7 x 10-8 6.6 x 10-7 ± 1.7 x 10-7 ∆H -1.2 x 104 ± 1.4 x 103 -1.7 x 103 ± 1.1 x 102 -2.0 x 103 ± 1.1 x 102 ∆S -12.6 22.2 21.7 target 8 kDa peptide 8 kDa peptide 8 kDa peptide stochiometry Table S1b. Isothermal titration calorimetry (ITC) parameters for bispecific MSA21FAF Nb1-3 FAF Nb1- FAF Nb2- FAF Nb2FAF Nb3-MSA21 MSA21 MSA21 MSA21 Na 0.9 ± 0.2 0.9 ± 2.8 x 10-2 0.8 ± 2.6 x 10-2 0.9 ± 9.4 x 10-2 Kd (M) 8.4 ± 3.3 x 10-7 6.9 ± 1.0 x 10-7 5.4 ± 0.6 x 10-7 7.5 ± 2.4 x 10-7 -1.0 x 104 ± -1.7 x 104 ± 7.1 ∆H a (-9.6 ± 2.3) x 10 2 -1.1 x 104 ± 1.7 x 103 4.6 x 102 x 102 ∆S 24.6 -6.06 -28.0 -8.38 target 8 kDa peptide 8 kDa peptide C68 8 kDa peptide stochiometry