ISMP Medication Safety Alert - Institute For Safe Medication Practices
advertisement

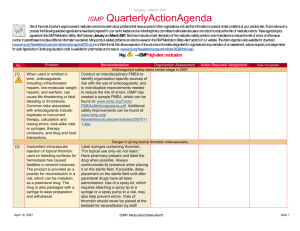
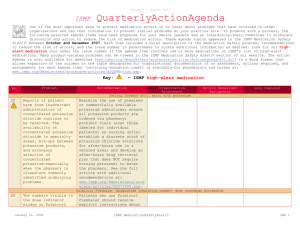
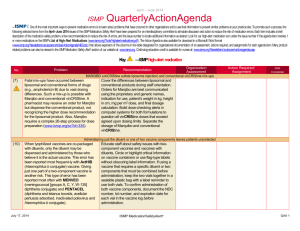
April - June 2013 ISMP QuarterlyActionAgenda One of the most important ways to prevent medication errors is to learn about problems that have occurred in other organizations and to use that information to prevent similar problems at your practice site. To promote such a process, the following selected items from the April-June 2013 issues of the ISMP Medication Safety Alert! have been prepared for an interdisciplinary committee to stimulate discussion and action to reduce the risk of medication errors. Each item includes a brief description of the medication safety problem, a few recommendations to reduce the risk of errors, and the issue number to locate additional information as desired. Look for our high-alert medication icon under the issue number if the agenda item involves one or more medications on the ISMP’s List of High-Alert Medications (www.ismp.org/Tools/highalertmedications.pdf). The Action Agenda is also available for download in a Microsoft Word format (www.ismp.org/Newsletters/acutecare/articles/Action Agenda1303.doc) that allows expansion of the columns in the table designated for organizational documentation of an assessment, actions required, and assignments for each agenda item. Many product-related problems can also be viewed in the ISMP Medication Safety Alert! section of our website at: www.ismp.org. Continuing education credit is available for nurses at: www.ismp.org/Newsletters/acutecare/actionagendas.asp. Key: No. Problem (11) An opioid-naïve surgical patient was started on morphine patient-controlled analgesia (PCA) with a 3 mg demand dose, 10 minute lockout, and a basal rate of 1 mg/hour. During the first night, the patient was found in respiratory distress and died. Gaps in patient monitoring, including a failure to employ pulse oximetry despite the presence of the equipment in the patient’s room, were the predominant factors, as was the failure to recognize progressive signs of impending respiratory depression. (7) —ISMP high-alert medication Organization Assessment Fatal PCA adverse events linked to inadequate monitoring Recommendation Action Required/ Assignment Date Completed Along with a basic physical assessment and vital signs, other parameters are vitally important when monitoring patients receiving PCA. The patient’s level of pain and sedation must be continually assessed; early recognition of excessive sedation and intervention is essential. Respiratory assessments should include evaluation of the rate, depth, quality of respiratory effort, pattern of respirations, and breath sounds. Technology to continuously monitor ventilation in patients known to be at risk for respiratory depression is recommended. High-alert medication list useless without bundled risk-reduction strategies Some hospitals have neither a wellPeriodically update your hospital-specific reasoned list of high-alert medications nor a list of high-alert medications, and robust process for managing the drugs on implement risk-reduction strategies that their list. The list may not be well known to address the underlying causes of errors, clinicians, and hospitals may rely on low which have been identified through a leverage risk-reduction strategies to prevent literature search, information from ISMP, errors. A list of high-alert medications is analysis of internal reports, and failure relatively useless unless it is up-to-date, mode and effects analysis (FMEA). Layer known by all clinical staff, and accompanied numerous risk-reduction strategies (see by vigorous risk-reduction strategies which Table in full article) that impact as many are more effective than awareness, manual steps of the medication process as double checks, and staff education. feasible. Assess the effectiveness of strategies. NAN Alert: Mix-ups between ado-trastuzumab emtansine (KADCYLA) and trastuzumab (HERCEPTIN) July 11, 2013 ISMP MedicationSafetyAlert! QAA 1 April – June 2013 ISMP Problem No. (8) The US Food and Drug Administration (FDA)-approved generic name for Kadcyla, ado-trastuzumab emtansine, may not be fully communicated when prescribed or may be read incompletely, thus risking confusion with trastuzumab. Given that the dosing and treatment schedules for these drugs are quite different, confusion could lead to dosing errors and potential harm to the patient. (12) As of May 2013, heparin labels must express the total amount of units in the container and the units per mL in parentheses, rather than just the units per mL. Before the change, the per mL quantity had been misinterpreted as the total amount per container, which led to overdoses and some fatalities. Errors are still possible during transition in which vials with the old and new label are both available, particularly as staff get used to the new label and then encounter a vial with the old label. (7) QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed Ensure that you are using the prefix “ado” with “trastuzumab” when referring to Kadcyla. Use both the brand and generic name when communicating orders on preprinted order sets or in computerized order entry systems for either of these drugs. Employ strategies to differentiate Kadcyla and Herceptin generic names and increase awareness of this issue. NAN Alert: Heparin label changes To minimize the risk of confusion, hospitals should consider transitioning fully to the newly labeled heparin, even if it means discarding some older vials. Otherwise, separate heparin vials with the old labeling from those with the new labeling, and use all vials with the old labeling first. As space permits, pharmacy and nursing databases should express drug amounts the same way as the vial label (i.e., 10,000 units/10 mL [1,000 units/mL]). Assessment shows widespread lack of knowledge regarding safe use of opioids A Pennsylvania Patient Safety Authority Based on the results of the opioid knowledge assessment of prescribers, knowledge assessment, organizations pharmacists, and nurses found that 78% should use a similar tool did not know a patient’s level of sedation (www.ismp.org/sc?id=205) to assess the was the most important predictor of understanding of prescribers, respiratory depression while receiving IV pharmacists, and nurses who care for opioids; 33% were unaware that 0.4 mg patients receiving opioids and provide of HYDROmorphone IV is equianalgesic staff education based on the findings to 2 mg of morphine IV. Other lowalong with other safeguards ISMP has scoring questions dealt with drugs that published (www.ismp.org/sc?id=210). potentiate opioid effects, the definition of an opioid-tolerant patient, and indications for using long-acting opioids. July 11, 2013 ISMP MedicationSafetyAlert! QAA 2 April – June 2013 ISMP Problem No. (8) (13) (10) (11) More than 1,100 patients may have received lower doses of cyclophosphamide and gemcitabine than prescribed. The amount of overfill in the containers was not clear, and nurses failed to infuse the entire volume in each bag (including overfill), resulting in administration of lower doses than intended. QuarterlyActionAgenda Organization Assessment Confusion regarding overfill with IV chemotherapy Recommendation Action Required/ Assignment Date Completed Establish a standard process to identify the overfill volume on the pharmacy label for compounded IV chemotherapy/biotherapy solutions. Ensure that nurses who administer these products can locate the final volume on the label, and can recognize that the full volume must be infused to administer the correct dose (unless otherwise stated). Mix-ups between leucovorin and LEVOleucovorin Two errors occurred at a hospital Separate the drugs wherever they are involving mix-ups between leucovorin stored. If using automated dispensing and LEVOleucovorin (FUSILEV) due to cabinets (ADCs), consider using tall man name similarity. There is significant lettering for LEVOleucovorin in ADC potential for dosing errors when software listings. Pop-up messages interchanging leucovorin and should also be considered, as they may LEVOleucovorin. The dose of be useful to educate, alert, and remind LEVOleucovorin is one-half the dose of staff of a possible mix-up. racemic leucovorin injection (leucovorin). Name confusion with new cancer drugs PAZOPanib (VOTRIENT) and PONATinib (ICLUSIG) Two new oral chemotherapy agents have Computer systems that list oral names that may cause confusion: chemotherapy linked to available dosage pazopanib and ponatinib. Tablet strengths will help to minimize the strengths and dosages are different, potential for error, as will listing which may help prevent and detect appropriate dosing information for errors. labeled indications. The use of tall man lettering with the unique letter characters differentiated (PONATinib and PAZOPanib) can also be helpful. The drug niMODipine is often given via a nasogastric tube. Unless pharmacy prepares the liquid and dispenses it in an oral syringe, nurses have to extract the product from the gel capsule with a syringe and needle. On at least 25 occasions, the liquid extracted from a July 11, 2013 IV administration of oral liquid niMODipine If pharmacy does not extemporaneously prepare a liquid formulation of niMODipine from capsules and dispense the drug in oral syringes, the hospital should use a new commercial product for adults, NYMALIZE (niMODipine) oral solution (www.ismp.org/sc?id=191). This ISMP MedicationSafetyAlert! QAA 3 April – June 2013 ISMP Problem No. QuarterlyActionAgenda Organization Assessment Recommendation capsule was accidentally injected IV, resulting in 5 deaths and 5 near-death events. product is indicated for improvement of neurological outcomes in adults with subarachnoid hemorrhage. (12) The value of a manual independent double check has been questioned by those who rarely find mistakes. Its use has been a source of stress for busy staff. Its overuse with high-alert drugs has been called to task given its status as a weak risk-reduction strategy, particularly if it’s the only safeguard in place. Its inconsistent use, variability with how it’s carried out, and the superficial manner in which many checks are conducted has rendered it less effective than anticipated. However, studies have shown that independent double checks can detect up to 95% of errors if done correctly. Independent double checks are undervalued and misused Evaluate the procedures for which you require a double check, monitor compliance, assess how often the checks are conducted as designed, and then make the necessary revisions to promote effectiveness. Manual double checks are most effective when conducted independently in a cognitive manner, and when used judiciously for selective high-risk tasks or high-alert medications. Avoid sole reliance on double checks as a safety strategy. Fewer double checks strategically placed at the most vulnerable points of the medication use process will be much more effective. (7) Three patients received nalbuphine instead of naloxone. One event involved the accidental selection of nalbuphine, which appeared immediately before naloxone on an automated dispensing cabinet (ADC) screen; another involved an ADC stocking error. The third error involved selection of the wrong product from an anesthesia tray. (9) British and Spanish authorities have analyzed 52 overdoses of IV acetaminophen in children younger than 1 year. Recently in the US, July 11, 2013 Action Required/ Assignment Date Completed Mix-ups between nalBUPHine and nalOXone Tall man lettering on ADC screens and pharmacy labels may help avoid mix-ups, as well as adding the words “rescue agent” next to nalOXone entries, if space permits. Locked lidded drawers for nalBUPHine and nalOXone could also prevent inadvertent selection of the wrong drug. 10-fold overdoses of IV acetaminophen When prescribing the drug, include the mg per kg weight-based dose along with the total calculated dose. For the product label and medication administration ISMP MedicationSafetyAlert! QAA 4 April – June 2013 ISMP Problem No. (10) (12) (7) QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed acetaminophen IV 250 mg was record, include both the mg dose and prescribed for a 25 kg child. A mental slip volume to be infused. Pharmacists led the clinician to program an infusion should prepare pediatric doses of 600 pump to deliver 250 mL (2,500 mg) mg or less in a syringe, and nurses instead of 250 mg (25 mL). Children and should administer the drug via a “smart” low-weight adults are at greatest risk of syringe pump with dose limits active in overdoses because they need less than the drug library. For adult infusions, a full 100 mL (1 g) bottle, thus requiring a smart pump use is also advised. calculation to determine the volume to infuse. Saline flush administered via ON-Q C-bloc during attempt to collect blood from central venous access device (CVAD) A laboratory technician who was Limit access to CVAD lines to those with attempting to collect blood from what she professional training. Promote a thought was a CVAD instead consistent process for tracing all administered a saline flush via a yellowcatheters/lines from the access site to an striped extension tubing connected to an infusion or capped access port before ON-Q C-bloc continuous peripheral nerve drawing blood, connecting tubing, or block infusion. The ON-Q system catheter administering drugs or solutions. Affix was anchored to the front of the patient’s labels on lines if the patient has more shoulder, and the upper part of the gown than one port of entry into the body. obscured the ON-Q system. A blue connector was visible and thought to be attached to the CVAD. Starter doses from the emergency department (ED) may not be dispensed in child-resistant containers EDs and physician practices often send Organizations that dispense drug samples patients home with drug samples or or starter doses should evaluate their starter doses in unit dose packages. practices and modify their procedures as These packages may not be child needed to ensure the safety of patients resistant. If dispensed in plastic bags or and children in their homes. With the envelopes, the medications may be easily increased number of 24-hour community accessed by children, especially if set pharmacies, these services may not be down on a counter upon arriving home necessary. from the ED. Mix-ups between risperiDONE and rOPINIRole Last year, ISMP and FDA received more When prescribing either drug, the purpose than 200 reports of mix-ups between of the drug should be included with the risperiDONE and rOPINIRole, and these order, and pharmacists and nurses should events have continued. In one of the verify that the therapy matches the latest cases, a patient experienced patient’s condition. In pharmacies, the nausea and an unexpected sedative products should not be stored near each July 11, 2013 ISMP MedicationSafetyAlert! QAA 5 April – June 2013 ISMP Problem No. effect after taking what was thought to be risperiDONE. Both products were from the same manufacturer and in the same shaped bottle with similar label printing. July 11, 2013 QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed other, and tall man lettering should be used for storage, labeling, and computer listings. ISMP MedicationSafetyAlert! QAA 6