ISMP Medication Safety Alert - Institute For Safe Medication Practices
advertisement

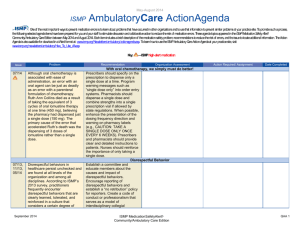
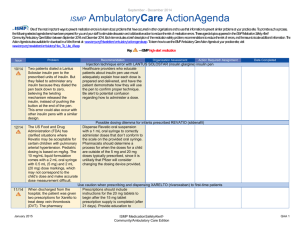
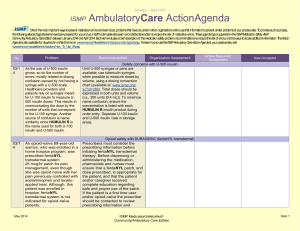
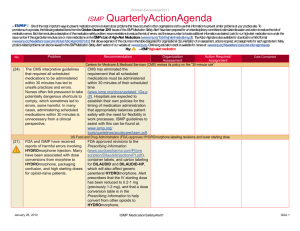
May - August 2012 ISMP AmbulatoryCare ActionAgenda Oneof themost important ways toprevent medication errors is tolearn about problems that haveoccurred in other organizations and tousethat information toprevent similar problems at your practicesite. To promotesuch aprocess, thefollowing selected agendaitems havebeen prepared for you and your staff tostimulatediscussion and collaborativeaction toreducetherisk of medication errors. Theseagendatopics appeared in theISMPMedication Safety Alert! Community/Ambulatory CareEdition between May 2012 and August 2012. Each itemincludes abrief description of themedication safety problem, recommendations toreducetherisk of errors, and theissuetolocateadditional information. TheAction Agendais alsoavailablefor download in aWord format (www.ismp.org/Newsletters/ambulatory/Issues/ActionAgenda201209.doc). Tolearn howtousetheISMPAmbulatory CareAction Agendaat your practice site, visit www.ismp.org/newsletters/ambulatory/How_To_Use_AA.asp. No. Problem 08/1 2 A community pharmacist inadvertently dispensed methadone to a 7-year-old boy who normally takes methylphenidate 10 mg BID. The child became lethargic and vomited after taking one dose, and the same symptoms occurred after giving the child the same dose a second time the following day. ISMP and the US Food and Drug Administration (FDA) have received multiple reports of confusion between methadone and methylphenidate. 07/1 2 We received yet another report of a mix-up between risperiDONE (RISPERDAL) and rOPINIRole (REQUIP). A patient experienced nausea and an unexpected sedative effect after taking what was thought to be risperiDONE. It was discovered that rOPINIRole tablets had been dispensed in error. As in earlier cases, the products were from the same manufacturer in the same shaped bottle with similar printing. September 2012 Recommendation Organization Assessment Action Required/ Assignment Date Completed Methadone or methylphenidate? Configure mnemonics in e-prescribing and pharmacy order entry systems to warn about confusion between methadone and other drugs that start with “meth” and have similar strengths. Separate methylphenidate and methadone in storage areas. Barcode scanning during the production stage of the dispensing process can identify when the wrong product is selected from the shelf. Identify barriers to successful scanning in order to minimize the risk of bypassing or overriding the scan of the product barcode. Mix-ups between risperiDONE and rOPINIRole The US Food and Drug Administration has asked manufacturers to use tall man lettering on container and carton labels. When prescribing either drug, the drug name should be printed and the purpose of the drug should be included on the prescription. In pharmacies, do not store the products near one another, and use tall man letters for storage labeling and computer listings. Implementing barcode scanning during the production stage of the dispensing process can identify wrong product selection from the shelf. ISMP MedicationSafetyAlert! Community/Ambulatory Care Edition QAA 1 May-August 2012 ISMP No. Problem 06/1 2 Upon listening to the pharmacy’s voice mail system, a licensed pharmacy intern questioned a prescription that sounded like “zolpidem 100 mg, take 4 tablets by mouth once daily.” The physician was contacted and intended for the patient to receive ZYLOPRIM (allopurinol) for gout. 05/1 2 An outreach worker visited the home of a patient with active tuberculosis to give a dose of isoniazid to the patient’s children. One of the children vomited after receiving one-half of the dose. The syringe containing the remainder of the dose was placed on a table while the child was cleaned. A sibling, who had already received his dose of medication, picked up the unsecured syringe and ingested the remainder of the medication. As a result, the child received approximately 800 mg of isoniazid in total. 07/1 2 Although medication samples may seem to help with the high cost of medications, there are many safety issues. First, they may be expired due to the lack of regular office staff audits, poor organization and inventory control, and storage space. September 2012 AmbulatoryCare ActionAgenda Recommendation Organization Assessment Action Required/ Assignment Date Completed Say what? ZYLOPRIM or zolpidem? Pharmacies should record information on their voice mail messaging system prompting prescribers to spell out the drug name and sound out the dose (e.g., one-five instead of fifteen), provide the indication, and use both brand and generic names when prescribing these drugs. Preventing unintentional mediation overdoses Keep all medications secured and out of the reach and sight of children, even when the caregiver is in the same area as the child. Teach children about medication safety, and tell them what medicine is and why their parent or caregiver must be the one to give it to them. For more information and strategies to protect children from unintentional medication overdoses, visit the Up and Away and Out of Sight educational campaign at: www.upandaway.org. Medication samples and safety concerns for physician practices Store medication samples in accordance with manufacturers’ labeling and in locked cabinets away from patient and staff traffic. Establish policies that indicate who is responsible to monitor storage, inspect for outdated product, maintain the sample medication log, and ISMP MedicationSafetyAlert! Community/Ambulatory Care Edition QAA 2 May-August 2012 ISMP AmbulatoryCare ActionAgenda Problem Recommendation Second, samples are distributed without safety checks that exist in pharmacies (e.g., computer screening for drug interactions, duplicate therapy, and contraindications). Lastly, patient education may be limited or narrow in scope and the samples may have problematic or confusing labeling. ensure safety measures are followed. Medication samples should be treated like new prescriptions with respect to screening for drug interactions, duplicate therapy, allergies, and contraindications. Pharmacists might be able to offer a consulting service to area physicians where they review office medication storage at regular intervals. No. 06/1 2 A mix-up of Sig codes was believed to be a cause for an error. During review of a refill for HYDROcodone and acetaminophen 5 mg / 500 mg, the directions printed on a label were “Test 4 times daily” instead of “Take 4 tablets daily.” The patient had been receiving the dosage for over a year so he continued to take the prescription correctly. The pharmacy’s investigation showed that the Sig code “T4TD” was used instead of “T4TDA,” which corresponded to the correct directions. 08/1 2 The manufacturer has halted distribution of individual EPIPEN (EPINEPHrine) 0.3 mg AutoInjectors and only distributes an EpiPen 2-Pak. The 2-Pak contains two EpiPens plus a training pen. The training pen, which looks like a real EpiPen, was found stored in the EpiPen drawer in an Emergency Department’s automated September 2012 Organization Assessment Action Required/ Assignment Date Completed Sig code shortcut to error Run reports of system Sig codes that are in use. These Sig codes should be evaluated and those that are at risk for causing an error should be removed. Furthermore, at the pointof-sale, pharmacy staff should review each prescription container with the patient, whether it is a new prescription or refill, by opening up the bag and prescription container. EpiPen 2-Pak plus training pen confusion Instruct patients and parents to separate training pens from the real thing and make sure that only the actual EpiPen is sent with children when they go back to school. Please be sure staff know about the risk of confusion between the training pen and actual pen. Long term care facilities should unpack the pens and store only the active pens in ADCs. ISMP MedicationSafetyAlert! Community/Ambulatory Care Edition QAA 3 May-August 2012 ISMP Problem No. AmbulatoryCare ActionAgenda Recommendation Organization Assessment Action Required/ Assignment Date Completed dispensing cabinet (ADC). 07/1 2 A nurse gave a patient 5 drams of acetaminophen concentrate liquid (100 mg/mL) instead of 5 mL. That’s equivalent to 18.45 mL, or 1.845 g of acetaminophen. The dose was measured in a dose cup with scales that measure in drams, fluid ounces, cc, mL, TSP, and TBSP. Also, the hospital was using concentrated acetaminophen instead of the new standard strength (160 mg/5 mL). 08/1 2 The Centers for Disease Control and Prevention has made a toolkit available to help educate healthcare providers and patients about safe injection practices. Any healthcare provider that gives injections should be aware of safe injection practices. 08/1 2 Keeping an up-to-date list of medications will help patients remember all the medications they are taking. Patients can share the list with doctors, hospital staff, and pharmacists, to help protect against mistakes. 06/1 2 ISMP has redesigned its medication safety website for consumers (www.consumermedsafety.org). There are many useful medication safety tools and resources available. September 2012 Archaic liquid measure a factor in medication errors Facilties should check supplies and remove measuring devices with archaic measurement scales. Pharmacists that serve a nursing home or other care facility, make sure these aren't in use. Also, pharmacists should not sell cups with archaic measurements. For liquid medications, oral syringes should be used instead of dose cups. Remove any concentrated acetaminophen from your supplies. Safe injection toolkit available The toolkit contains audio-visual and print materials that can be distributed at staff meetings, incorporated into inservice education, and posted in public areas. To access the toolkit, visit: www.oneandonlycampaign.org/conte nt/healthcareprovider-toolkit. Help your patients keep an up-to-date list of medications ISMP has developed a Personal Medicine Form, available at: www.ismp.org/Tools/personal_med_f orm. Please share the form with your patients. ISMP consumer website updated Consider how the website might benefit your patients. Please help ISMP promote the website by sharing the link in consumer information that you provide. Please also include the link on your website. ISMP MedicationSafetyAlert! Community/Ambulatory Care Edition QAA 4 May-August 2012 ISMP September 2012 AmbulatoryCare ActionAgenda ISMP MedicationSafetyAlert! Community/Ambulatory Care Edition QAA 5