to open the MS Word version of the Quarterly Action Agenda
advertisement

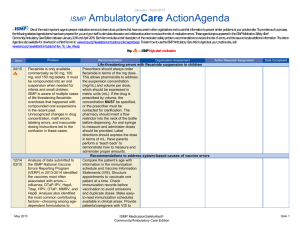
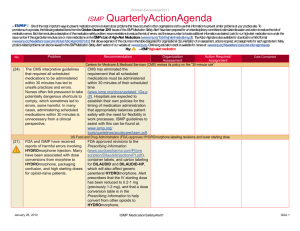
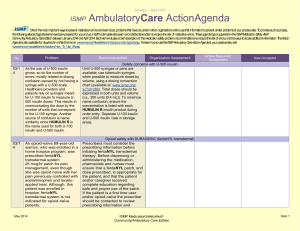
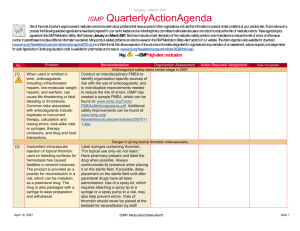
April – June 2014 ISMP QuarterlyActionAgenda One of the most important ways to prevent medication errors is to learn about problems that have occurred in other organizations and to use that information to prevent similar problems at your practice site. To promote such a process, the following selected items from the April—June 2014 issues of the ISMP Medication Safety Alert! have been prepared for an interdisciplinary committee to stimulate discussion and action to reduce the risk of medication errors. Each item includes a brief description of the medication safety problem, a few recommendations to reduce the risk of errors, and the issue number to locate additional information as desired. Look for our high-alert medication icon under the issue number if the agenda item involves 1 or more medications on the ISMP’s List of High-Alert Medications (www.ismp.org/Tools/highalertmedications.pdf). The Action Agenda is also available for download in a Microsoft Word format (www.ismp.org/Newsletters/acutecare/articles/ActionAgenda1403.doc) that allows expansion of the columns in the table designated for organizational documentation of an assessment, actions required, and assignments for each agenda item. Many productrelated problems can also be viewed in the ISMP Medication Safety Alert! section of our website at: www.ismp.org. Continuing education credit is available for nurses at: www.ismp.org/Newsletters/acutecare/actionagendas.asp. Key: Problem No. (7) (10) —ISMP high-alert medication Organization Assessment MARQIBO (vinCRIStine sulfate liposome injection) and conventional vinCRIStine mix-ups Fatal mix-ups have occurred between liposomal and conventional forms of drugs (e.g., amphotericin B) due to vast dosing differences. Such a mix-up is possible with Marqibo and conventional vinCRIStine. A pharmacist may receive an order for Marqibo but dispense the conventional product, not recognizing the higher dose recommendation for the liposomal product. Also, Marqibo requires a complex 26-step process for dose preparation (www.ismp.org/sc?id=335). Recommendation Action Required/ Assignment Date Completed Cover the differences between liposomal and conventional products during staff orientation. Orders for Marqibo are best communicated using the proprietary and generic names, indication for use, patient’s weight in kg, height in cm, mg per m2 dose, and final dosage calculation. Build dose-checking alerts in computer systems for both formulations to question all vinCRIStine doses that exceed agreed upon dosing limits. Separate the storage of Marquibo and conventional vinCRIStine. Administering just the diluent or one of two vaccine components leaves patients unprotected When lyophilized vaccines are co-packaged Educate staff about safety issues with twowith diluents, only the diluent may be component vaccines and vaccines with dispensed and administered by those who diluents. Circle or highlight critical information believe it is the actual vaccine. This error has on vaccine containers or use flag-type labels been reported most frequent-ly with ActHIB without obscuring label information. If using a (Haemophilus b conjugate) vaccine. Giving vaccine that requires a specific diluent or two just one part of a two-component vaccine is components that must be combined before another risk. This type of error has been administration, keep the two vials together in a reported most often with MENVEO sealable plastic bag with a label reminder to (meningococcal [groups A, C, Y, W-135] use both vials. To confirm administration of diphtheria conjugate) and PENTACEL both vaccine components, document the NDC (diphtheria and tetanus toxoids, acellular number, lot number, and expiration date for pertussis adsorbed, inactivated poliovirus and each vial in the vaccine log before Haemophilus b conjugate). administration. July 17, 2014 ISMP MedicationSafetyAlert! QAA 1 April – June 2014 ISMP Problem No. Organization Assessment ACTIQ (fentaNYL citrate oral transmucosal lozenge) mistaken as throat lozenge (9) Three providers ordered Actiq to treat a sore throat, mistaking the powerful opioid for a typical throat lozenge. All of the patients were opioid naïve, but serious harm was avoided when pharmacists detected the error before dispensing the product. (11) A patient experienced seizures and a cardiac arrest after receiving magnesium sulfate instead of 0.45% sodium chloride injection with 20 mEq of potassium chloride. Pharmacy had recently added 40 g magnesium sulfate bags to an automated dispens-ing cabinet (ADC) in an area that previously held bags of 0.45% sodium chloride injection with 20 mEq of potassium chloride. The nurse had difficulty reading the red-font labels through an overwrap. (12) QuarterlyActionAgenda Recommendation Action Required/ Assignment Date Completed Prescribing Actiq should be limited to pain management specialists, anesthesiologists, oncologists, palliative care, and hospice providers who review an educational program (www.ismp.org/sc? id=357) and complete a knowledge assessment required by the drug’s Risk Evaluation and Mitigation Strategy (REMS). Limit magnesium sulfate premix to 20 g bags If needed, magnesium sulfate premixed solutions should be stocked in units using the 20 g/500 mL bags, not the 40 g/1,000 mL bags. The smaller volume helps limit the amount of magnesium a patient might receive if a rapid infusion occurs accidentally. In the ED, hospitals may be able to stock only bags intended for bolus doses (e.g., 2 g/50 mL, 4 g/100 mL). Some organizations use the 4 g minibags for maintenance infusions. Some IV medications are diluted unnecessarily in patient care areas, creating undue risk An ISMP survey of nearly 1,800 nurses found Conduct a nursing survey to learn the extent that most dilute certain IV push medications for and variability of dilution practices. Conduct adults prior to administration. Even prefilled educational programs to dispel myths and help syringes con-taining a patient-specific dose are nurses see the risk associated with diluted by almost a quarter of nurses. unnecessary dilution. When possible, require Unnecessary dilution often leads to unlabeled pharmacy to prepare any IV push medications or mislabeled syringes, potential conthat must be diluted according to the tamination of sterile IV medications, dosing manufacturer’s guidelines or hospital policy. If errors, and other problems. Many nurses also sta-bility requires dilution immediately prior to reported the oft-unnecessary practice of administration, provide directions for dilution withdrawing a medication from a prefilled via written or electronic guidelines or checklists syringe and further diluting it in a larger syringe that provide standard diluent volumes and for patients with an implanted port or a concentrations. peripherally inserted central catheter (PICC). July 17, 2014 ISMP MedicationSafetyAlert! QAA 2 April – June 2014 ISMP No. Problem 201 3: Disrespectful behaviors in healthcare persist uncheck-ed and are found at all levels of the organization and among all disciplines. According to ISMP’s 2013 survey, practitioners frequently encounter disrespectful behaviors that are clearly learned, tolerated, and reinforced in a culture that considers a certain degree of disrespect to be a “normal” style of interaction. Productivity demands, cost containment, and hierarchies that nurture a sense of status and autonomy have been the most influential factors. Disrespectful behaviors cause the recipient to experience fear, vulnerability, anger, humiliation, uncertainty, and self-doubt. The behaviors erode professional communication and collaboration, and have been linked to adverse events, even patient mortality. (13, 20) 201 4: (8) (7) Currently, Luer type connectors on medical tubing, ports, and catheters have a universal design that allows misconnections between devices that serve completely different functions (e.g., enteral tubing connected to a tracheostomy tube inflatable cuff or an IV line). Tubing misconnections are rare, but when they occur, patient injuries can be serious, lifethreatening, and even fatal. QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed Disrespectful behaviors Establish a committee and educate members about the causes and impact of disrespectful behavior. Encourage reporting of disrespectful behaviors and establish a “no retribution” policy for reporters. Create a code of conduct or professionalism that serves as a model of interdisciplinary collegial relationships and collaboration. Establish a communication strategy for staff who must convey important information to enhance approachability and reduce intimidating behaviors. Establish an escalation policy to manage conflicts about the safety of an order when the standard communication process fails. Develop an intervention policy that has leadership support to consistently address disrespectful behavior. New connectors coming for enteral feeding tubes New enteral feeding device connectors that will not allow connectivity to any other type of connector will be introduced later this year. New enteral-specific administration sets will be available in the last quarter of 2014. Enteral syringes for flushing and boluses will be available with the new con-nector in the first quarter of 2015, and new enteral feeding tubes will be available in the second quarter. Visit Stay Connected 2014 (www.staycon nected2014.org) to prepare for these changes. Guide patients to visit ConsumerMedSafety.org July 17, 2014 ISMP MedicationSafetyAlert! QAA 3 April – June 2014 ISMP Problem No. (7) Educating hospitalized patients about safe medication practices can be a daunting task given their short length of stay, limited attention span when ill, and other health and safety topics of importance that require patient education. Because patients often seek information about health and safety online, hospitals can help patients reduce the risk of a medication error by guiding patients to reputable online resources. (12) Phenylephrine hydrochloride injection (10 mg/mL) 1 mL vials are often available for emergency use. However, bolus doses (50250 mcg) cannot be drawn up accurately from a 10 mg vial, and, therefore, dilution is needed. Directions for dilution are not on the label, and the package insert may not be handy in an emergency. (10) (10) QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed Health systems with a website accessible to patients should link to ISMP’s ConsumerMedSafety.org, a user-friendly, online resource that imparts knowledge about safe medication practices in ways that consumers can easily access, view, and use. The real-world content can be searched by topic or drug. Medication safety stories, overthe-counter medicine and insulin safety sections, a medication safety toolbox, and a consumer medication error-reporting system are examples of the content areas. Phenylephrine injection needs dilution for IV bolus Create a dilution guideline or checklist for this drug according to the package insert for diluting 10 mg (1 mL) to yield a 100 mcg/mL concentration for bolus doses. If possible, have pharmacy attend codes to properly prepare this and other emergency medications as needed for bolus dosing. TASIGNA (nilotinib) label instructions may conflict with prescriber instructions Confusion may lead to dosing errors with ISMP informed FDA and Novartis about the Tasigna because printed instructions on the inci-dent and asked for removal of the dosing manufacturer’s packaging reflect ONLY the instructions from the blister packages. Prior to recommended starting dose. One patient took patient discharge or dispensing a prescription, two 200 mg capsules twice daily, as written on reinforce the correct dosing instructions and the packaging, rather than once daily (400 tell the patient if it differs from what is mg), as written on the prescription label. The suggested on the blister pack. Give the patient patient developed QT prolongation. correct written instructions. An order was placed for posaconazole 200 mg PO TID with meals, but no dosage form was July 17, 2014 Posaconazole (NOXAFIL) dose depends on dosage form If both oral products (oral suspension, delayed-release tablets) are available at ISMP MedicationSafetyAlert! QAA 4 April – June 2014 ISMP QuarterlyActionAgenda Organization Assessment Problem Recommendation specified. The prescriber thought an oral suspension would be used since, in the past, that had been the only dosage form available. In 2013, delayed-release tablets were made available (and now there is an injectable form). The order was transcribed as delayed-release tablets. This is clinically relevant since the delayed-release tablets have substantially higher bioavailability than the oral suspension. your institution, you may want to install a clinical alert to appear at the time the drug is ordered as a reminder of the various dosage forms. No. Action Required/ Assignment Date Completed Vancomycin injection for oral use given IM Pharmacies should prepare the injectable solution for oral use and provide each individual dose in an oral syringe marked “FOR ORAL USE ONLY.” Dispensing medications in the most ready-toadminister form should be the prevailing practice for all pharmacies that provide medications in hospitals and LTC facilities. (12) Due to the high cost of vancomycin capsules, the injectable form of vancomycin powder is often prepared as an oral solution to treat C. difficile. In two long-term care (LTC) facilities, the pharmacy provided vials of vancomycin injection and diluent with directions for mixing and administering each dose. Nurses were unfamiliar with this practice and administered each dose intramuscularly (IM), rendering it ineffective to treat C. difficile. (12) When electronically prescribing Brintellix 10 mg daily for a patient with major depressive disorder, a physician incorrectly selected Brilinta, an antiplate-let agent. The patient picked up the filled prescription but realized, after reading an attached drug information leaflet, that a mistake had been made. If these drugs are available at your facility or associated outpatient locations, take steps to reduce the risk of confusion, including building alerts to remind prescribers to use generic names (in addition to brand names) and list the indication when prescribing these drugs. (11) Problems may arise during written or electronic communication because of similarities in appearance of the alphanumeric symbols we use. For example, the letter “l” can look like the Misidentification of alphanumeric symbols Promote visibility, legibility, and readability in written communication using the following methods: use lowercase or mixed-case letters to provide distinction; encourage prescribers to clearly print handwritten orders; provide lightly BRINTELLIX (vortioxetine) and BRILINTA (ticagrelor) drug name confusion July 17, 2014 ISMP MedicationSafetyAlert! QAA 5 April – June 2014 ISMP Problem No. numeral “1,” especially when the drug name and dose are close together like “Levoxyl25 mg.” There are similar issues with other letters and numerals that share similar characteristics. July 17, 2014 QuarterlyActionAgenda Organization Assessment Recommendation Action Required/ Assignment Date Completed lined order forms to prevent interference with symbols; be selective about font and typeface; avoid italics and underlining; and ensure proper spacing between drug names and doses, and between numerical doses and the unit of measure. ISMP MedicationSafetyAlert! QAA 6