Bio 110 Exam I Study Guide
advertisement

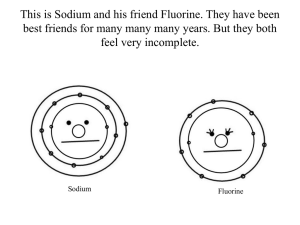
Biology 110 Bio 110 Exam I Study Guide Chapter 1 1. Define the following: a. Abdominal cavity b. Abdominopelvic cavity c. Anatomy d. Cranial cavity e. Disease f. Distal g. Frontal plane h. Homeostasis i. Lateral j. Lumbar k. Medial l. Mediastinum m. Netative feedback n. Organ transplantation o. Pelvic cavity p. Pericardial cavity q. Physiology r. Pleural cavity s. Proximal t. Sagittal plane u. Spinal cavity v. Systemic disease w. Thoracic cavity x. Transverse plane y. Visceral 2. How is the structure of an organ related to its function? Give an example. 3. What are the levels of structural organization of the body? 4. What is a tissue? 5. How many tissues make up an organ? 6. What is an organ system? 7. What is the standard anatomical position? 8. Know all the planes and what you get when you slice a body up through each one 9. Know all those directional terms (relative positions of body parts). 10. What do you find in the axial portion of the body? 11. What do you find in the appendicular portion of the body? 12. List the cavities of the body and what they contain. 13. What smaller cavities are found in each? 14. What are the 9 anatomically defined abdominopelvic regions? 15. …and those quadrants? 16. What is homeostasis? 17. How is it maintained (homeostasis)? 18. How do negative and positive feedback mechanisms work? Biology 110 Chapter 2 Elements and Atoms Name and describe the subatomic particles of an atom, and indicate which one accounts for the occurrence of isotopes. 19. 20. 21. 22. 23. What is a proton? What is its charge? What is its mass? Where is it found? What is a neutron? What is its charge? What is its mass? Where is it found? What is an electron? What is its charge? What is its mass? Where is it found? What is the definition of atomic number? In an element the number of electrons is equal to the number of what other subatomic particle? 24. What is the atomic mass of an atom equal to? 25. What is an isotope? Molecules and Compounds Distinguish between ionic and covalent reactions, and between ionic and covalent bonds. List and discuss the functions of ions in the body. 26. What is an ion? 27. How is an ion formed? 28. Why is an ion formed? 29. What is the rule of eights? 30. When ions are formed are electrons donated, accepted, or shared? 31. How are covalent bonds formed? 32. Are the electrons that form a covalent bond shared equally? 33. What is the difference between a polar and nonpolar covalent bond? 34. What is a hydrogen bond? 35. Which type of bond is the strongest? Which type is the weakest? 36. Fluorine has the atomic number 9, sodium has the atomic number 11. a. What is the number of protons in each element? b. The atomic mass of fluorine is 19 and sodium 23. What would you guess the number of neutrons to be in each element? c. How many electrons would each element have? d. Knowing that having 8 electrons in the outer shell will make the element more stable, which of the following correctly describes the way fluorine and sodium will form a bond: i. Fluorine will donate a proton to sodium ii. Sodium will donate a proton to fluorine iii. Fluorine will donate a neutron to sodium iv. Sodium will donate a neutron to fluorine v. They will share 8 electrons to form a covalent bond vi. Sodium will donate an electron to fluorine, and become an ion vii. Sodium will accept an electron from fluorine, and become an ion viii. Fluorine will donate an electron to sodium, and become an ion ix. Fluorine will accept an electron from sodium, and become an ion Biology 110 x. The ions will be attracted because of their charge difference and form an ionic bond 37. When chlorine accepts an electron to become an ion what charge does the chlorine ion have and why? 38. What is the difference between an element and a molecule and a compound? 39. Can an element have different kinds of atoms (don’t count isotopes)? 40. How about a molecule? 41. Does a compound have different kinds of molecules? 42. Can a compound have different kinds of atoms? 43. Will an ionic bond break easily in water? Will a covalent bond do the same thing? Some Important Inorganic Molecules 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. Describe the structure of water, and give examples of how it functions in the body. Relate the term electrolyte to the presence of ions in the body fluids and tissues. Define the terms acid and base. Describe the pH scale, and explain the significance of buffers. Why is water a polar molecule? What properties does the molecular structure of water allow it to have? What is cohesion? What does it allow water to do? Why is a high heat of vaporization good? Why is water a good solvent? What does a non-polar compound do in water? Does water dissociate? Into what? What is a condensation synthesis (dehydration) reaction? What is a hydrolysis reaction? What is an electrolyte? What is the difference between an acid and a base? How is acidity measured? Would a solution with a pH of 6.5 be acidic, basic, or neutral? What is the pH of a neutral solution? What is acidosis? What is alkalosis? What is a buffer? Some Important Organic Molecules Compare and contrast the structures and functions of carbohydrates, lipids, proteins, and nucleic acids. Explain what enzymes are, and describe their role in the body. Describe how the structure and function of DNA and RNA differ. Describe the structure of ATP, and explain how ATP functions in the body. 61. Identify which of the following are lipids, which are carbohydrates, which are proteins, and which are nucleic acids: i. Fatty acids ii. Glycogen iii. Triglycerides Biology 110 iv. Cholesterol v. Phospholipids vi. Glucose vii. Sucrose viii. Monosaccharides ix. Polysaccharides x. Glycerol xi. DNA xii. RNA xiii. Enzymes xiv. Chains of amino acids 62. Which of the following contains nitrogen: carbohydrates, fats, proteins, nucleic acids 63. What are the functions of carbohydrates? 64. What are the building blocks of carbohydrates? 65. What is a polysaccharide? 66. What are the functions of lipids? 67. What are the components of triglycerides? 68. What are steroids? 69. What are phospholipids? What does the phosphate group do for the lipid? 70. What are the functions of proteins? 71. What are the building blocks of proteins? 72. What kind of bond links the subunits of proteins? 73. What is the difference between a structural and functional protein? 74. What are the four levels of protein structure? 75. What are enzymes? 76. What is denaturation? What kinds of things cause it? 77. What are the 3 basic building blocks of a nucleotide? 78. What are the rules for hydrogen bonding between nitrogenous bases? 79. What is the difference between DNA and RNA? Yes, of course I mean both structurally and functionally. 80. Which nucleotides are found in DNA? How about RNA? 81. What is a gene? 82. If a section of a DNA strand has the bases ATTGACT what bases will the opposite strand have? 83. What is special about ATP? (What does it do?) Chapter 3 84. What are the structural components of the plasma membrane? 85. What is a phospholipid bilayer? 86. What does cholesterol do for the plasma membrane? 87. What is the function of proteins in the plasma membrane? 88. What is the role of sugar residues on the plasma membrane? 89. What are the majopr components of the nucleus? 90. What is the difference between chromatin and chromosomes? 91. What is the function of messenger RNA? 92. What is a mutation? 93. What is the function of the nucleolus? 94. What is the cytoplasm primarily composed of? Biology 110 95. What is the function of a ribosome? 96. What is transfer RNA? 97. What is ribosomal RNA? 98. What is the endoplasmic reticulum (ER)? 99. What is the difference between smooth and rough ER? 100. What are peroxisomes? 101. What is the Golgi apparatus 102. What are lysomes? 103. What are mitochondria? 104. What are centrioles? 105. What is the difference between cilia and flagella? 106. What is the difference between active and passive transport? 107. Define diffusion, osmosis, and filtration. 108. Where does the energy for active transport come from? 109. What is endocytosis? 110. What is the difference between pinocytosis and phagocytosis? 111. What is exocytosis? 112. What are the two types of cell division and what is the difference between them? Chapter 4 113. What are the four major types of tissues? 114. What functions may epithelial tissues perform? 115. How may epithelial tissues be classified (shape and layers)? 116. What is function of connective tissue? 117. What are the components of connective tissue? (cells and matrix, which may have fibers) 118. What is the difference between loose and fibrous connective tissue? 119. What is cartilage? How is it classified? 120. What is special about the matrix of bone? 121. What is the matrix of blood? (yeah, it’s liquid, but it also is not made by the “connective tissue” cells themselves) 122. What is muscular tissue composed of? 123. What are the major filaments in muscle? 124. What are the 3 types of muscle and where are they found? 125. What is nervous tissue? 126. What types of cells are found in nervous tissue? 127. What are the different types of membranes? 128. What organs or structures is each type of membrane associated with? 129. What are the functions of each type of membrane?