Lung function and bronchodilator response in 4-year
advertisement

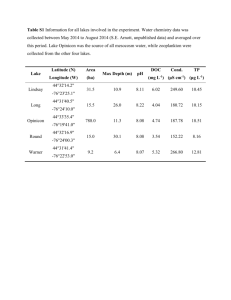
Lung function and bronchodilator response in 4-year-old children with different wheezing phenotypes Ellie Oostveen, Sandra Dom, Kristine Desager, Margo Hagendorens, Wilfried De Backer, Joost Weyler Online Depository Methods Study design and study population The Prospective Cohort on the Influence of Perinatal Factors on the Occurrence of Asthma and Allergies (PIPO) study is a prospective birth cohort study involving 1128 children (1). Between June 1997 and December 2001, pregnant women in the Antwerp region in Belgium were invited to participate by completing a screening questionnaire. Data on demography, respiratory symptoms and risk factors for asthma were collected through the use of postal questionnaires completed by the parents: the first questionnaire covered the first year of life, with subsequent questionnaires at 6-month intervals up to the age of 4 years. All questionnaires were based on validated questionnaires from the International Study of Asthma and Allergies in Childhood (ISAAC) (2). In addition to the questionnaires, the children were invited to participate in a medical examination at the University of Antwerp Hospital at the ages of 1 and 4 years. Both the parents and the participating child were asked to provide a blood sample for allergy testing. At the age of 4 years, the children were also invited for lung function testing by the forced oscillation technique as part of the medical examination. Atopic sensitisation Total and specific IgE levels were assessed from blood samples from the children and their parents, taken when the children were aged 1 and/or 4 years. IgE was determined via a fluorescence enzyme immunoassay technique (CAP FEIA systems, Phadia, Brussels, Belgium). In the 1-year-old children, specific IgE against 1 Dermatophagoides pteronyssinus, cat, dog, hen’s egg and cow’s milk were determined, while in the 4-year-old children, specific IgE levels against birch pollen and timothy grass pollen were also quantified. In the parents, specific IgE levels against 7 common inhalant allergens (D. pteronyssinus, cat, dog, birch pollen, timothy grass pollen, mugwort pollen and Cladosporium herbarum) were quantified. Specific IgE levels ≥ 0.35 kU.L-1 were considered positive. Atopic sensitization of the child/mother was defined as at least one positive specific IgE at the age of 1 or 4 years. Definitions of wheezing phenotypes: Symptoms of wheeze were assessed through the answers to the core questions from the International Study of Asthma and Allergies in Childhood questionnaire (wheeze during the past 6 (or 12) months and the number of wheezing episodes) (2). On the basis of the history of wheeze reported by the parents, the children were divided into the following wheezing phenotypes (3), (4): - ‘never wheeze’: children who had never wheezed during the first 4 years of life; - ‘early-transient wheeze’: at least 1 wheezing episode during the first 3 years of life, but no wheezing in the fourth year; - ‘late-onset wheeze’: no wheeze in the first 3 years of life, and at least 1 episode of wheeze in the fourth year. - ‘persistent wheeze’: at least 1 episode of wheeze in the first 3 years, and at least 1 episode of wheeze in the fourth year. 2 Forced oscillation technique A custom-made forced oscillation setup was used to measure the respiratory impedance (Zrs) in the 4-year-old children (See Figure 1). The setup and Zrs measurements met the requirements of the ATS and ERS recommendations (5, 6). A loudspeaker generated a pseudorandom noise excitation pressure in the frequency range 4 to 32 Hz ( 2 Hz) with 1 cmH2O of amplitude. Pressure and flow signals were measured with identical Honeywell pressure transducers and a Fleisch no. 1 pneumotachograph. Since the breathing circuit was continuously flushed with a bias flow, no bacterial filter was used. The setup including the mouth piece had a dead space of 35 mL. The measured impedance data were corrected for the impedance of the pneumotachograph. Pressure and flow signals were low-passfiltered and recorded for 16 s, time-averaged with time blocks of 0.5 s, and Fourieranalyzed to obtain Zrs as the complex ratio between the pressure and the flow signal. At least 5 measurements were collected, the child coming off the mouthpiece in between the measurements. In the event of large within-test variation, Zrs data were re-analyzed retrospectively. The average of 3-5 acceptable Zrs data was used for further analysis. Reasons for the discarding of data were air leakage, swallowing, glottis closure during the actual measurement, or outlying data relative to the average data set. Power analysis In a large Antwerp epidemiological study performed some 10 years ago (7), it was observed that 20% of the 5-7-year-old children had experienced “ever wheeze”, 50% 3 of whom had had complaints of wheeze in the past twelve months. Our extrapolation of these findings leads us to expect that 10% of the 4-year-old children had experienced wheeze in the past 12 months. n1: total population n2: group of children with wheeze n2/n1 = = 0.1 Furthermore, we anticipate that 4-year-old children with wheeze have a > 10% higher airway resistance relative to the normals (4) |1- 2|: hypothetical expected difference between the groups; |1- 2| = 0.10) and that the variation of airway resistance in healthy preschool children is ~20% [(8); R5= 1.03 ± 0.23 kPa.s.L-1 (population standard deviation: = 0.2)]. Power (1-) = 0.80. The formula (where a one-tailed = 0.05 was assumed) (n1+n2) ≥ ((1+ )2/ ) × (z0.05 + z)2 × 2 /(1- 2)2 + (z0.052)/2 ≥ ((1 + 0.1)2/ 0.1) × (z0.05 + z)2 × 2 /(1- 2)2 + (z0.052)/2 ≥ 12.1 × 6.19 × 4 + 1.4 ≥ 300 indicates that a total sample size of about 300 children should be sufficient to detect differences in lung function between children with and without wheeze. Data analysis Exclusion of Zrs data In some children, huge variations between the individual measurements were observed in Zrs, and especially in Rrs. We arbitrarily defined a Zrs measurement as 4 reliable when the coefficient of variation (CoV= SD/mean value) of the Rrs data points was < 15% in more than half of the data points of that particular test. With this reliability criterion, we excluded the Zrs data from 6 children at baseline; postbronchodilator Zrs data were rejected in another 10 children. Results The success rate of FOT in these 4-year-old children was high: acceptable Zrs data were attained in 95% of the children. The coefficients of variation (CoVs) of the baseline respiratory resistance at 4, 6 and 8 Hz (R4, R6 and R8) for the overall cohort (n=535) were on average 8.6% (SD 4.3), 7.7% (3.1) and 7.7 % (3.1), respectively. The average CoVs of R4, R6 and R8 for the groups of children with different wheezing phenotypes were not meaningfully different from each other and were always below 10%. The within-test variabilities of the baseline reactance data at 4, 6 and 8 Hz (X4, X6 and X8) were significantly lower than those of R4, R6 and R8, respectively (p<0.001 at all frequencies, paired t-test). In more than one-third of the children who underwent lung function testing (see Figure 2), the wheezing phenotype of the child could not be determined because questionnaires had not been adequately completed or had not been returned. In our study design, postal questionnaires were sent out every 6 months. Despite the small numbers of children in the different wheezing phenotype groups, significant differences were observed both in baseline respiratory function and in the 5 bronchodilator response between the children with different wheezing phenotypes. At 4 years of age, the children with early-transient wheeze yielded higher baseline resistance values as compared with those who never wheezed. The children with persistent wheeze displayed a poorer baseline lung function than that of the children who never wheezed or those with early-transient wheeze, and their bronchodilator response was larger. Adjustment for potential confounders Mean resistance and reactance values at 4, 6 and 8 Hz and the area under the reactance curve (AX, see text) were estimated by multiple linear regression (MLR) analysis with the sex, age, weight, height, education level of the mother, atopy of the mother, presence of siblings, exposure to parental smoking, pets, eczema, rhinitis, atopy of the child, lower respiratory tract infections and antibiotics use at 4 years of age as potential confounders. These analyses did not alter the results. Exclusion of the children who used inhaled corticosteroids at 4 years of age (n=8) or who presented with common cold at the test day (n=45) did not alter the results of the analyses either. We also investigated whether atopy per se explains the group differences in lung function. The separate MLR analysis where the atopy of the child, the wheeze phenotype and the lung function indices were taken into account, revealed that the associations between all the lung function indices and the wheeze phenotype became even stronger on the addition of atopy to the model, except for fres and fres, where the association was left unchanged. 6 Table 1. Baseline resistance and reactance values and the absolute and relative changes with respect to the baseline after bronchodilation in the groups of children with different wheezing phenotypes Baseline R4 (hPa.s.L-1) R6 (hPa.s.L-1) R8 (hPa.s.L-1) X4 X6 X8 AX fres (hPa.s.L-1) (hPa.s.L-1) (hPa.s.L-1) (hPa.L-1) (Hz) Bronchodilation R4 R6 R8 R4 R6 R8 X4 X6 X8 AX fres X4| X6| X8| AX| (hPa.s.L-1) (hPa.s.L-1) (hPa.s.L-1) (% baseline) (% baseline) (% baseline) (hPa.s.L-1) (hPa.s.L-1) (hPa.s.L-1) (hPa.L-1) (Hz) (% baseline) (% baseline) (% baseline) (% baseline) Never Early Persistent n=144 n=127 n=54 mean (95% CI) mean (95% CI) 10.3 (9.9 , 10.7) 11.0 (10.5 , 11.5) * 11.9 (11.1 , 12.7) ***, † 9.8 (9.5 , 10.2) 10.5 (10.1 , 11.0) * 11.5 (10.7 , 12.2) ***, †† 9.6 (9.2 , 9.9) 10.1 (9.7 , 10.6) 11.0 (10.3 , 11.7) ***, † -4.1 (-4.4 , -3.9) -4.5 (-4.8 , -4.2) -4.8 (-5.3 , -4.3) * -2.6 (-2.7 , -2.4) -3.0 (-3.2 , -2.7) * -3.3 (-3.7 , -2.9) ** -1.5 (-1.7 , -1.4) -2.0 (-2.3 , -1.8) ** -2.3 (-2.8 , -1.9) ** 19.9 (17.6 , 22.1) 27.2 (23.4 , 31.1) ** 32.6 (25.8 , 39.3) *** 16.2 (15.5 , 17.0) 18.2 (17.3 , 19.1) ** 19.5 (17.9 , 21.0) *** n=121 n=53 n=139 mean (95% CI) -2.3 (-2.6 , -2.0) -2.6 (-3.1 , -2.2) -3.4 (-4.1 , -2.7) *, † -2.1 (-2.4 , -1.9) -2.5 (-2.9 , -2.2) -3.2 (-3.8 , -2.5) ** -2.2 (-2.4 , -1.9) -2.4 (-2.8 , -2.1) -3.1 (-3.7 , -2.5) **, † -21.5 (-23.8, -19.2) -22.1 (-24.6, -19.5) -26.0 (-30.1, -25.9) -20.7 (-22.9, -18.4) -22.3 (-25.0, -19.7) -25.4 (-29.4, -21.3) * -21.4 (-23.7, -19.1) -22.6 (-25.2, -20.0) -26.1 (-30.0, -22.2) 1.0 (0.9 , 1.2) 1.2 (1.0 , 1.5) 1.4 (1.0 , 1.8) 0.9 (0.8 , 1.0) 1.1 (1.0 , 1.3) 1.4 (1.0 , 1.8) * 0.9 (0.7 , 1.0) 1.2 (1.0 , 1.4) 1.5 (1.1 , 1.9) ** -9.8 (-11.6 , -8.1) -16.3 -4.4 (-5.1 , -3.7) -5.5 (-6.3 , -4.8) * -6.8 (-8.2 , -5.3) 23.0 (19.8, 26.1) 24.2 (21.2, 24.1) 25.2 (19.9, 30.4) 33.2 (29.6, 36.9) 35.2 (31.6, 38.7) 36.1 (28.8, 43.4) 57.2 (46.7, 67.7) 60.1 (48.2, 72.0) 63.7 (44.8, 82.6) 43.0 (37.2, 48.8) 50.9 (47.0, 54.9) 53.9 (46.5, 61.4) ** (-19.6 , -13.0) ** -21.2 (-27.4 , -15.1) *** ** * R4, R6, R8 and X4, X6, X8: resistance and reactance, respectively, at 4, 6 and 8 Hz. 7 AX: area under the reactance curve. fres: resonance frequency. CI: confidence interval. *, **, ***: p< 0.05, 0.01 and 0.001, respectively, relative to children who never wheezed. †, ††: p< 0.05 and 0.01, respectively, relative to children with earlytransient wheeze. 8 Table 2. Cutoff values for a significant bronchodilator response based on the 5th (95th) percentile in the group of children who never wheezed. Absolute change Relative change |R4| 5.5 (hPa.s.L-1) 43 %baseline |R6| 5.0 (hPa.s.L-1) 41 %baseline |R8| 5.2 (hPa.s.L-1) 43 %baseline |X4| 2.7 (hPa.s.L-1) 51 %baseline |X6| 2.3 (hPa.s.L-1) 62 %baseline |X8| 2.4 (hPa.s.L-1) 120 %baseline |AX| 31 (hPa.L-1) 81 %baseline 9 BF PN MP LS PT Figure 1. Forced oscillation setup for measurement of respiratory impedance. MP: mouth piece, PN: pneumotachograph, PT: pressure transducers, BF: bias flow, LS: loudspeaker. 10 Invited for medical examination and LF testing (n=1128) Medical examination performed (n=577) No permission for LF testing (n=10) Permission given for LF testing (n=567) FOT successful (n=543) Included in study Excluded from study: (n=535) medication use (n=2) FOT not successful (n=24) - equipment failure (n=5) - reluctance of the child (n=8) - artifacts during measurement (n=11) unreliable data (n=6) Study population (n=332) Incomplete questionnaires (n=203) Figure 2: Flow chart of the process of data collection in the children participating in the medical examination and lung function (LF) testing at 4 years of age. 11 References 1. Hagendorens MM, Bridts CH, Lauwers K, van Nuijs S, Ebo DG, Vellinga A, De Clerck LS, Van Bever HP, Weyler JJ, Stevens WJ. Perinatal risk factors for sensitization, atopic dermatitis and wheezing during the first year of life (PIPO study). Clin Exp Allergy 2005; 35: 733-740. 2. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Resp J 1995; 8: 483-491. 3. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. New Engl J Med 1995; 332: 133-138. 4. Brussee JE, Smit HA, Koopman LP, Wijga AH, Kerkhof M, Corver K, Vos APH, Gerritsen J, Grobbee DE, Brunekreef B, Merkus PJFM, de Jongste JC. Interrupter resistance and wheezing phenotypes at 4 years of age. Am J Respir Crit Care Med 2004; 169: 209-213. 5. Oostveen E, MacLeod D, Lorino H, Farré R, Hantos Z, Desager K, Marchal F. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Resp J 2003; 22: 1026-1041. 6. Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, Gappa M, Gaultier C, Gustafsson PM, Hall GL, Hantos Z, Healy MJ, Jones MH, Klug B, Lødrup Carlsen KC, McKenzie SA, Marchal F, Mayer OH, Merkus PJ, Morris MG, Oostveen E, 12 Pillow JJ, Seddon PC, Silverman M, Sly PD, Stocks J, Tepper RS, Vilozni D, Wilson NM. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007; 175: 1304-1345. 7. Wieringa MH, Vermeire PA, Van Bever HP, Nelen VJ, Weyler JJ. Higher occurrence of asthma-related symptoms in an urban than a suburban area in adults, but not in children. Eur Resp J 2001; 17: 422-427. 8. Hellinckx J, De Boeck K, Bande-Knops J, van der Poel M, Demedts M. Bronchodilator response in 3-6.5 years old healthy and stable asthmatic children. Eur Respir J 1998; 12: 438-443. 13