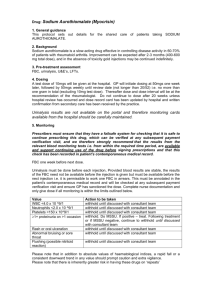
Shared Care Form
advertisement

NHS Grampian Nationally Enhanced Service Shared Care Policy and Prescribing Information for General Practitioners for METHOTREXATE PATIENT NAME UNIT NUMBER CHI NUMBER HOSPITAL WARD TELEPHONE NO CONSULTANT ADDRESS DATE OF BIRTH Insert patient sticker here THERAPEUTIC INDICATION FOR THIS PATIENT: SIGNATURE DATE (to be completed by consultant) DOSAGE/PREPARATION/ROUTE/FREQUENCY OF ADMINISTRATION: Starting dose of 7.5mg as a single oral dose ONCE WEEKLY Concurrent Folic Acid Y / N * Dose = 5mg (oral) ONCE WEEKLY (72 hours after Methotrexate) *Delete where appropriate. CARE WHICH IS THE RESPONSIBILITY OF THE HOSPITAL CONSULTANT Baseline: Chest X-ray, FBC, U & E, and LFTs. Copy of results to be sent to GP. CARE WHICH IS THE RESPONSIBILITY OF THE GENERAL PRACTITIONER 1. Prescribing of medication. 2. The General Practitioner has primary responsibility for monitoring therapy according to the schedule below: PIIINP test (dermatology only) – twice prior to treatment then repeated at 4 monthly intervals. FBC, U&E and LFT (incl. ALT and Alk Phos) fortnightly until 6 weeks after the last dose increase Initiation of therapy and recommendations for dose increments. Decision on final dose FBC, U&E and LFT monthly while on stable dose required. Increase monitoring to 2 weekly after any change in dose. Monitoring clinical response to treatment. Patients should be asked about the development of any new or increasing fever, dyspnoea or cough or the (If agreed by both Consultant and GP blood presence of rash or oral ulceration at each visit. request forms may be endorsed (by the GP) so that a copy is sent to the Consultant who will When writing laboratory request make a secondary check on the results.) forms always include details of However normal practice is that the clinician the patient’s medication who orders the test must act on the result. NOTE: in addition to absolute values for haematological indices a rapid fall or a consistent downward trend in any value should prompt caution and extra vigilance. What to do if something unexpected occurs Contact consultant ADDITIONAL INFORMATION: (to be completed by consultant where necessary) Please keep this document in the patients notes Page 1 of 2 NHS Grampian Nationally Enhanced Service Shared Care Policy and Prescribing Information for General Practitioners for METHOTREXATE MONITORING: - Action to be taken if: WBC <4.0x109/L Neutrophils <2x109/L Platelets <150x109/L >2 fold rise in ALT or Alk. Phos (from upper limit of reference range) Unexplained fall in albumin: Rash or oral ulceration: New or increasing dyspnoea or cough: Unexplained fever: withhold until discussed with consultant Withhold until discussed with consultant MCV>105fl investigate and if B12 or folate low start appropriate supplementation Significant deterioration in renal function: discuss with consultant Abnormal bruising or sore throat withhold until FBC result available OTHER INFORMATION Low dose aspirin and standard doses of NSAIDs may be continued Co-trimoxazole or trimethoprim must be avoided in patients taking methotrexate Live vaccines should be avoided in patients taking methotrexate - annual influenza vaccine should be given Methotrexate is available in strengths of 2.5mg and 10mg - ensure that the patient is aware of which strength(s) have been prescribed Responsibilities of GPs undertaking monitoring A GP agreeing to monitor methotrexate should: Ensure that the necessary blood tests are normal Ensure that the relevant blood monitoring requirements are undertaken, and at the correct frequency Ensure that the blood test results are checked for any abnormality at the same frequency as the tests are being undertaken Only continue to prescribe methotrexate if it is being satisfactorily monitored Contact a consultant in the event of a drug reaction or monitoring abnormality Be alert for any of the known adverse reactions. The patient should be encouraged to ensure blood tests are taken at the correct intervals. FOR SPECIFIC PRODUCT INFORMATION PLEASE CONSULT THE CURRENT SUMMARY OF PRODUCT CHARACTERISTICS Page 2 of 2