Capillary Electrophoresis and Capillary Electrochromatography.
advertisement

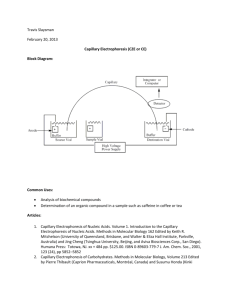
Capillary Electrophoresis and Capillary Electrochromatography. Chapter 30 An Overview of Electrophoresis. Electrophoresis is a separation method based on the differential rate of migration of charged species in a buffer solution across which has been applied a dc electric field. Positively charged ions migrate towards a negative electrode and negatively charged ions migrate towards a positive electrode. For safety reasons one electrode is usually at ground and the other is biased positively or negatively. Ions have different migration rates depending on their total charge, size, and shape, and can therefore be separated. This separation technique was first developed by the Swedish chemist Arne Tiselius in the 1930s for the study of serum proteins; he was awarded the 1948 Nobel Prize for this work. Electrophoresis has been applied to a variety of difficult analytical separation problems: inorganic anions and cations, amino acids, catecholamines, drugs, nucleic acids, nucleotides, polynucleotides, and numerous other. A particular strength of electrophoresis is its unique ability to separate charged macromolecules of interest in the biotechnology industry and in biochemical and biological research. For many years, electrophoresis has been the powerhouse method of separation of proteins (enzymes, hormones, antibodies) and nucleic acids (DNA, RNA) for which it offers unparalleled resolution. An electrophoretic separation is performed by injecting a small band of the sample into an aqueous buffer solution that is contained in a narrow tube on a flat porous support medium such as paper or a semisolid gel. A high de potential is applied across the length of the buffer by means of a pair of electrodes located at either end of the buffer. This potential causes ions of the sample to migrate towards one or the other of the electrodes. 30A-1 Types of Electrophoresis Electrophoretic separations are currently performed in two quite different formats: one is called slab electrophoresis and the other capillary electrophoresis. Slab separations are carried out on a thin flat layer or slab of a porous semisolid gel containing an aqueous buffer solution within its pores. Ordinarily this slab has dimensions of a few centimeters on a side and, like a chromatographic thin layer plate, is capable of separating several samples simultaneously. Slab electrophoresis is currently the most widely used separation tool of the biochemist and the biologist. Capillary electrophoresis, which is an instrumental version of electrophoresis, has been developed and used only in the last decade and one half and has become an important separation tool used by chemists and life scientists. In many cases, this new method of performing electrophoretic separations appears to be a satisfactory substitute for slab electrophoresis with several important advantages, which will be described subsequently. 30A-2 The Basis for Electrophoretic Separations The migration velocity v of an ion in centimeters per second in an electric field is equal to the product of the field strength E (Vcm –1) and the electrophoretic mobility ue(cm2V-1s-1). That is v = ue E The electrophoretic mobility is in turn proportional to the ionic charge on the analyte and inversely proportional to frictional retarding factors. The electric field acts only on ions. 30B) Capillary Electrophoresis. As useful as conventional slab electrophoresis has been, and continues to be, this type of electrophoretic separation is typically slow, labor intensive, difficult to automate, and does not yield very precise quantitative information. Capillary electrophoresis yields high-speed, high-resolution separations on exceptionally small sample volumes (0.1 to 10nL in contrast to slab electrophoresis, which requires samples in the uL range). 30B-1 Migration Rates and Plate Heights in Capillary Electrophoresis. As we have already seen in the previous equation, an ion’s migration velocity v depends upon the electric field strength. The electric field in turn is determined by the magnitude of the applied potential (V, in volts) and the length L over which it is applied. Thus, v = ue (V/L) This relationship indicates that high-applied potentials are desirable to achieve rapid ionic migration and a rapid separation. It is desirable to have rapid separations, but it is even more important to achieve high-resolution separation. So we need to examine the factors that determine the resolution in electrophoresis. 30B-2 Plate Heights in Capillary Electrophoresis. In chromatography, both longitudinal diffusion and mass-transfer resistance contribute to band broadening. However, since only a single phase is involved for electrophoresis, only longitudinal diffusion need be considered. It has been shown for electrophoresis, that the plate count (N) is given by N = (ue*V/2D) Where D is the diffusion coefficient of the solute in cm2s-1. Because resolution increases as the plate count increases, it is desirable to use highapplied potentials in order to achieve high-resolution separation. 30B-3 Electroosmotic Flow When a high potential is applied across a capillary tube containing a buffer solution, electroosmotic flow usually occurs, in which the solvent migrates toward the cathode or the anode. The rate of migration can be appreciable. For example, a 50-mM pH 8 buffer has been found to flow through a 50cm capillary toward the cathode at approximately 5cm/min with an applied potential of 25KV. As shown in Figure 30-2 above, the cause of electroosmotic flow is the electric double layer that develops at the silica/solution interface. The rate of electroosmotic flow is generally greater than the electrophoretic migration velocities of the individual ions and effectively becomes the mobile-phase pump of capillary zone electrophoresis. Even though analytes migrate according to their charges within the capillary, the electroosmostic flow rate is usually sufficient to sweep all positive, neutral, and even negative species toward the same end of the capillary, so that all can be detected as they pass by a common point. The resulting electropherogram looks just like a chromatogram. The electroosmostic flow velocity v is given by the- equation V = ueoE In the presence of electroosmosis, an ion’s velocity is the sum of its migration velocity and the velocity of electroosmostic flow. Thus, V = (ue + ueo ) E Note that ue for an anion will carry a negative sign. 30B-4 Instrumentation for Capillary Electrophoresis. As shown below, the instrumentation for capillary electrophoresis is simple. A buffer-filled fused-silica capillary, that is typically 10 to 100 um in internal diameter and 40 to 100 cm long, extends between two buffer reservoirs that also hold platinum electrodes. Sample introduction is performed at one end, and detection at the other. The polarity of the highvoltage power supply can be as indicated, or can be reversed to allow rapid separation of anions. Although the instrumentation is conceptually simple, there are significant experimental difficulties in sample introduction and detection due to the very small volumes involved. Since the volume of a normal capillary is 4 to 5 uL, injection and detection volumes must be on the order of a few nanoliters or less. Sample Introduction: The most common introduction methods are electokinetic injection and pressure injection. With electrokinetic injection, one end of the capillary and its electrode are removed from their buffer compartment and placed in a small cup containing the sample. A potential is then applied for a measured time, causing the sample to enter the capillary by a combination of ionic migration and electroosmotic flow. Detection. Because the separated analytes move past a common point in most types of capillary electrophoresis, detectors are similar in design and function to those described for HPLC. One difference in behavior of detectors is encountered, however, because in capillary electrophoresis each ion migrates at a rate determines by its electrophoretic mobility. Thus, analyte bands passes through the detector at different rates, which results in peak areas that are somewhat dependent upon retention times. In contrast, in HPLC all species pass through the detector at the velocity of the mobile phase, and peak areas are independent of retention times. Ordinarily this time dependence is of no concern. Table 30-1 lists the detection methods that have been reported through 1988 for capillary electrophoresis. The second column of the table lists representative detection limits for these detectors. Absorbance Methods. Both fluorescence and absorbance detectors are widely used in capillary electrophoresis, although the latter are more common because they are more generally applicable. In order to improve the sensitivity of absorbance measurements, several techniques have been suggested for increasing the path length of the measurements. Indirect Detection. Indirect absorbance detection has been used for detection of species that are difficult to detect because of low molar absorptivities without derivatizaton. An ionic chromophore is placed in the electrophoresis buffer. The detector then receives a constant signal due to the presence of this substance. Fluorescence Detection. Just as in HPLC, fluorescence detection yields increased sensitivity and selectivity for fluorescent analytes or fluorescent derivatives. Laser-based instrumentation is preferred in order to focus the excitation radiation on the small capillary and to achieve the low detection limits available from intense source. Laser fluorescence detection has allowed detection of only 10 zeptomoles or 6000 molecules. Electrochemical Detection. Two types of electrochemical detection have been used with capillary electrophoresis: conductivity and amperometry. One of the problems with electrochemical detection has been that of isolation the detector electrodes from the high potential required for the separation. One method for isolation involves inserting of a porous glass or graphite joint between the end of the capillary and second capillary containing the detector electrodes. Mass Spectrometric Detection. The very small volumetric flow rates of under 1 uL .min from electrophoresis capillaries makes it feasible to couple the effluent from the capillary of an electrophoretic device directly to the ionization source of a mass spectrometer. Currently the most common sample introduction/ionization interface used for this purpose is electrospray, although fast atom bombardment has also been applied. Capillary electrophoresis with mass spectrometric detection is only a decade and a half old but has become of major interest to biologists and biochemists for the determination of large molecules that occur in nature, such as proteins, DNA fragments, and peptides. 30C APPLICATION OF CAPILLARY ELECTROPHORESIS. Capillary electrophoretic separations are performed in several ways called modes. It is noteworthy that these modes were first employed in slab electrophoresis and were subsequently adapted for capillary electrophoretic separations. These modes include capillary zone electrophoresis (CZE), capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), and capillary isotachophoresis (CITP). 30C-1 Capillary Zone Electrophoresis. In capillary zone electrophoresis (CZE), the buffer composition is constant throughout the region of the separation. 30C-2 Capillary Gel Electrophoresis. Capillary gel electrophoresis (CGE) is generally performed in a porous gel polymer matrix, the pores of which contain a buffer mixture in which the separation is carried out. 30C-3 Capillary Isotachophoresis. In capillary isotachophoresis (CITP) all analyte bands ultimately migrate at the same velocity; hence, the name from iso for same and tach for speed. 30D CAPILLARY ELECTROCHROMATOGRAPHY. Electrochromatography is a hybrid of capillary electrophoresis and HPLC that offers some of the best features of the two techniques. Two types of capillary electrochromatography have been developed since the early 1980s: packed column and micellar electrokinetic capillary. Capillary electrochromatography CEC appears to offer several advantages over either of the parent techniques. First, like HPLC, it is applicable to the separation of uncharged species. Second, like capillary electrophoresis, it provides highly efficient separations on microvolumes of sample solution without the need for a high-pressure pumping system. In electrochromatography, a mobile phase is transported through a stationary phase by electroosmostic-flow pumping rather than by mechanical pumping, thus simplifying the system significantly. References: http://www.acs.org http://www.cas.org http://www.chemcenter/org http://www.sciencemag.org http://www.kerouac.pharm.uky.edu/asrg/wave/wavehp.html http://www.scimedia.com/chem-ed/sep/electrop/electrop.htm http://www.scimedia.com/chem-ed/sep/electrop/cap-el.htm