tut11-04
advertisement

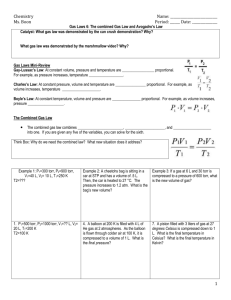
Tutorial 11 1. The Ideal Gas Law e.g. A steel cylinder is filled with 150 mol Argon gas at 25oC and 7.5 MPa. Some of the gas is used, then the pressure is 1.2 MPa at 17oC. What mass of gas remains in the cylinder? The volume of the cylinder is V = nRT/P = 150 mol(8.314 J K-1 mol-1)(298 K) / 7.5 x 106 Pa = 0.0496 m3 After some gas is removed, the number of moles remaining is n = PV/RT = 1.2 x 106 Pa(0.0496 m3)/(8.314 J K-1 mol-1)(290 K) = 24.7 mol e.g. A compressed gas cylinder at 13.7 MPa and 23oC is in a room where a fire raises the temperature to 950oC. Find the pressure at the new temperature. According to the laws of Boyle and Charles: P1V1/T1 = P2V2/T2 The volume of the cylinder does not change, so P1/T1 = P2/T2, or P2 = P1(T2/T1) = 13.7 MPa (1223/296) = 56.6 MPa e.g. A hot air balloon is filled to a volume of 4.0 x 103 m3 at 745 Torr and 21oC. The air is then heated to 62oC, causing the balloon to expand to a volume of 4.2 x 103 m3. Find the ratio of the number of moles of air in the heated balloon to the unheated balloon. P1V1/n1T1 = P2V2/n2T2 Thus, n2/n1 = P2V2T1/P1V1T2. Note that the internal pressure of the balloon remains constant, since it is open to the atmosphere at the bottom where the hot gases are blown in. Thus, P2 = P1, and n2/n1 = V2T1/V1T2. n2/n1 = 4.2 x 103 m3 (294 K) / (4.0 x 103 m3 (335 K)) = 0.92 2. Partial Pressures e.g. A mixture of 1.00 g H2 and 1.00 g He exerts a pressure of 0.480 atm. What is the partial pressure of each gas in the mixture? nH2 = 1.00 g / 2.02 g/mol = 0.495 mol nHe = 1.00 g / 4.00 g/mol = 0.250 mol XH2 = .495/(.495 + .250) = 0.66 XHe = .250/(.495 + .250) = 0.34 PH2 = XH2 (Ptot) = 0.66(0.48) = 0.317 atm PHe = XHe (Ptot) = 0.34(0.48) = 0.163 atm e.g. Helium gas is collected over water at 25oC and 1 atm total pressure. What volume of wet gas must be collected to obtain 0.586 g He? At 25oC, the vapor pressure of water is 23.8 Torr. If the total pressure is 1 atm, the partial pressure of Helium must be 1 atm - (23.8/760) = 0.969 atm. The number of moles of helium is 0.586 g / 4.00 g/mol = 0.1465 mol. Thus, the volume is V = nRT/P = 0.1465 mol(0.082 L atm K-1 mol-1)(298 K) / 0.969 atm = 3.69 L. 3. Gas Density, Reaction Stoichiometry e.g. SiCl4 is used to make electronics grade silicon. Calculate the density of the pure gas at 85oC and 758 Torr. 758 Torr = (758/760) atm x 101,325 Pa/atm = 101058 Pa = MW(P)/(RT). Thus, for SiCl4, MW = 28.1 + 4(35.5) = 170.1 g/mol = 0.170 kg/mol. Thus, = 0.170 kg/mol(101058 Pa)/(8.314 J K-1 mol-1 x (273+85)K)) = 5.77 kg/m3 = 5.77 g/L e.g. What mass of Helium is needed to fill a 1.5 L balloon at STP? There are two ways to solve this one. First, we could calculate the density as above, and second we could use the molar volume of a gas at STP, which is 22.4 L. Using the first method, = MW(P)/(RT) = 0.004 kg/mol(101325 Pa)/(8.314 J K-1 mol-1 x (273K) = 0.179 kg/m3 = 0.179 g/L. Thus, a 1.5 L balloon contains 1.5 L x 0.179 g/L = 0.269 g He Using the other method, the balloon must contain 1.5 L / 22.4 L mol-1 = 0.06696 mol He. This has a mass of 0.06696 mol x 4.00 g/mol = 0.268 g He. e.g. In 1897 the Swedish explorer Andree tried to reach the North Pole using a hydrogen filled baloon. He filled it with hydrogen using the reaction Fe(s) + H2SO4(aq) FeSO4(aq) + H2(g) The volume of the balloon was estimated as 4800 m3 and the loss of hydrogen during filling was estimated as 20%. What mass of iron and 98% H2SO4(aq) were required to fill the balloon? Assume 0oC and 1.00 atm during filling (i.e. STP). The volume of hydrogen required was 4800 m3 plus 25% = 6000 m3 = 6,000,000 L. At STP, this volume of gas is 6,000,000 L / 22.4 L mol-1 = 267,900 mol H2(g) This would thus require the same number of moles of iron and sulphuric acid. Thus, the mass of iron required was 267,900 mol x 55.8 g mol-1 / 1000 g kg-1 = 14,950 kg iron And the mass of acid required was 267,900 mol x 98 g mol-1 / 0.98 / 1000 = 26,790 kg 98% sulphuric acid. Kinetic Molecular Theory, Effusion, Diffusion e.g. Calculate the average velocity of gaseous Hg atoms at 25oC and at 500oC. v = [3RT/MW]1/2 At 25oC (298 K), the average velocity will be v = [3(8.314 J K-1 mol-1 x (273+25)K / 0.2006 kg mol-1]1/2 = 192.4 m/sAt 500oC (773 K), the average velocity will be v = [3(8.314 J K-1 mol-1 x (273+500)K / 0.2006 kg mol-1]1/2 = 310.0 m/s e.g. Is it easier to separate the oxygen isotopes 12C and 16C by diffusion using CO2 or CO? This is an application of Graham's Law. The easier separation process will have a ratio of diffusion rates that is larger than the other. Thus, fewer diffusion stages would be required. For the isotopes as the dioxides, we have: rate(12CO2) / rate(16CO2) = [MW(16CO2) / MW(12CO2)]1/2 = [48.0 / 44.0]1/2 = 1.044 rate(12CO) / rate(16CO) = [MW(16CO) / MW(12CO)]1/2 = [32.0 / 28.0]1/2 = 1.069 Thus, separation of the monoxides would be easier. Still easier to separate would be the methanes of the two carbon isotopes: rate(12CH4) / rate(16CH4) = [MW(16CH4) / MW(12CH4)]1/2 = [20.0 / 16.0]1/2 = 1.118