Gas Laws Review: Boyle's, Charles', Ideal Gas Law
advertisement

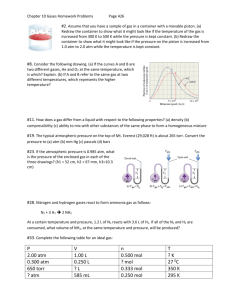
Ch 13 Review Gases Most important information to know from the chapter. Do not forget that you are also responsible for the calculations and concepts in the Molar Volume of Hydrogen Gas Lab. 13.1 Describing the Properties of Gases The common units for pressure are mm Hg (torr), atmosphere (atm), and pascal (Pa). The SI unit is the pascal Boyle’s Law states that the volume of a given amount of gas at a constant temperature varies inversely to it pressure. PV = k Charles’ law states that the volume of a given amount of an ideal gas at constant pressure varies directly with its temperature (in kelvins). V = bT o At absolute zero (0 K; -273 oC) the volume of an ideal gas extrapolates to zero Avogadro’s law states for an ideal gas at constant temperature and pressure, the volume varies directly with the number of moles of gas (n). V= an 13.2 Using Gas Laws to Solve Problems The ideal gas law describes the relationship among P, V, n, and T for an ideal gas. PV = nRT o R = (0.08206 L atm/mol K) o A gas that obeys this law exactly is called an ideal gas From the ideal gas law we can obtain the combined gas law which applies when n is constant o P1V1/T1 = P2V2/T2 Dalton’s law of partial pressure states the total pressure of a mixture of gases is equal to the sum of the individual (partial) pressures of the gases. o P total = P1 + P2 + P3 + … Standard temperature and pressure (STP) is defined as P = 1 atm and T = 273 K (0 oC) The volume of one mole of an ideal gas (the molar volume) is 22.4 L at STP 13.3 Using a Model to Describe Gases The kinetic molecular theory (KMT) is a model based on the properties of individual gas components that explains the relationship of P, V, T, and n for an ideal gas A law is a summary of experimental observations A model (theory) is an attempt to explain observed behavior The temperature of an ideal gas reflects the average kinetic energy of the gas particles The pressure of a gas increases as its temperature increases because the gas particles speed up The volume of a gas must increase because the gas particles speed up as a gas is heated to a higher temperature REVIEW QUESTIONS! Remember the steps! Step 1 – Make a table Step 2 – Determine the relationships and formula(s) Step 3 – Check all your units, plug in and solve 1. For each of the following sets of pressure and volume data, calculate the missing quantity. Assume that the temperature and the amount of gas remain the same. a. V = 291 mL at 1.07 atm; V = ? at 2.14 atm b. V = 1.25 L at 755 mm Hg; V = ? at 3.51 atm c. V = 2.71 L at 101.5 kPa; V = 3.00 L at ? mm Hg 2. For each of the following sets of volume and temperature data, calculate the missing quantity. Assume that the pressure and the mass of gas remain constant. a. V = 2.01 X 102 L at 1150 oC; V = 5.00 L at ? oC b. V = 44.2 mL at 298 K; V = ? at 0 K c. V = 44.2 mL at 298K; V = ? at 0 oC 3. If 0.214 mol of argon gas occupies a volume of 652 mL at a particular temperature and pressure, what volume would 0.375 mol of argon occupy under the same conditions? 4. What will be the new volume if 125 mL of He gas at 100. oC and 0.981 atm is cooled at 25 oC and the pressure is increased to 1.15 atm? 5. A tank contains a mixture of 3.0 mol N2, 2.0 mol O2, and 1.0 mol CO2 at 25 oC and a total pressure of 10.0 atm. Calculate the partial pressure (in torr) of each gas in the mixture. 6. If water is added to magnesium nitride, ammonia gas is produced when the mixture is heated. Mg3N2(s) + 3H2O(l) 3MgO(s) + 2NH3(g) If 10.3 g of magnesium nitride is treated with water, what volume of ammonia gas would be collected at 24 oC and 752 mm Hg? 7. Potassium permanganate, KMnO4, is produced commercially by oxidizing aqueous potassium manganate, K2MnO4, with chlorine gas. The unbalanced chemical equation is K2MnO4(aq) + Cl2(g) KMnO4(s) + KCl(aq) What volume of Cl2(g), measured at STP, is needed to produce 10.0 g of KMnO4?