Supplementary Figure 1 Fig. S1: Total viable protoplast yield from
advertisement

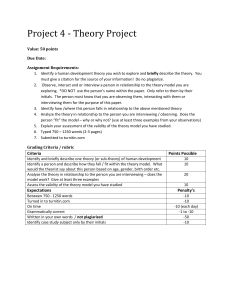
Supplementary Figure 1 Fig. S1: Total viable protoplast yield from 50mL enzyme digest as a function of incubation period. The optimal yield was obtained earliest at 12 hours of incubation under conditions used. Error bars represent standard deviation from the mean, n= 3. 1 Supplementary Figure 2 A B Fig. S2: (A) FDA staining of protoplasts to examine viability via confocal microscopy, (B) differential interference contrast field view of protoplasts from A. Bar = 100µm 2 Supplementary Data Appendix 1 Salt Medium (Modified KM Medium) This protoplast-sourced callus regeneration medium is adapted from that described by Kao and Michayluk (1975) (1) KM Mineral salts (mg/l) – from Duchefa, Belgium (dry powder) (2) Vitamins (mg/l) – from Duchefa, Belgium (1000X stock added after autoclaving) (3) Glucose (6.8% w/v) & Mannitol (4% w/v) (4) Sugars and sugar alcohols Sucrose Fructose Ribose Xylose Mannose 1X 250 250 250 250 250 100 X Stock 50mL (mg) 1250 1250 1250 1250 1250 (5) Organic acids & Addenda (pH adjusted to 5.5 with NH4OH) Sodium pyruvate Citric acid Casein hydrolysate (Enzymatic) D-Calcium Pantothenate (6) 2,4-D BAP NAA 1X 20 40 250 1 0.2 mg/L 0.5 mg/L 1.0 mg/L 1X Rhamnose Cellobiose Sorbitol Mannitol 250 250 250 250 50mL (mg) 1250 1250 1250 1250 100 X Stock 50mL (mg) 100 Malic acid 200 Fumaric acid 1250 Folic acid Ascorbic acid 5 Choline Cl Riboflavin 1X 40 40 0.4 2.0 1.00 0.20 50mL (mg) 200 200 2 10 5 1 (stock prepared earlier) (stock prepared earlier) (stock prepared earlier) To make 1000mL of KM (KOM) Medium: To dry weighed KM basal salts, glucose, mannitol and vitamin powders add 10mL of Sol 4, and 10mL of Sol 5. Add 980mL dH20. The pH of the medium is set to 5.6 (using NaOH). The medium is autoclaved before use. 3