CB1 receptor western blotting
advertisement
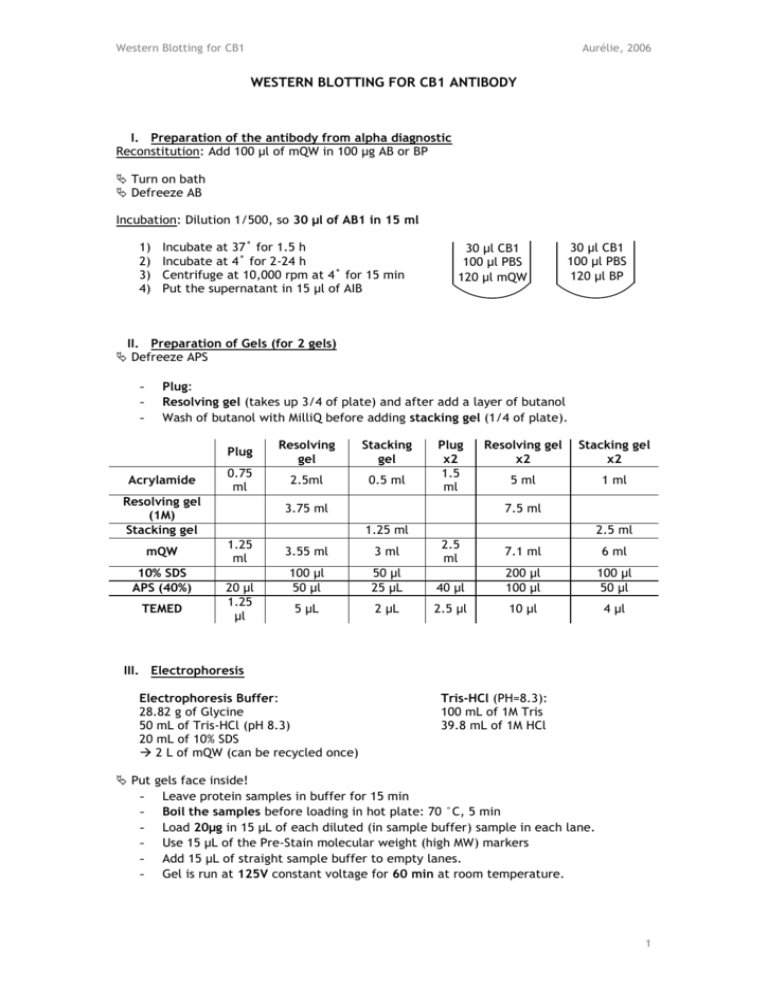
Western Blotting for CB1 Aurélie, 2006 WESTERN BLOTTING FOR CB1 ANTIBODY I. Preparation of the antibody from alpha diagnostic Reconstitution: Add 100 µl of mQW in 100 µg AB or BP Turn on bath Defreeze AB Incubation: Dilution 1/500, so 30 µl of AB1 in 15 ml 1) 2) 3) 4) Incubate at 37˚ for 1.5 h Incubate at 4˚ for 2-24 h Centrifuge at 10,000 rpm at 4˚ for 15 min Put the supernatant in 15 µl of AIB 30 µl CB1 100 µl PBS 120 µl mQW 30 µl CB1 100 µl PBS 120 µl BP II. Preparation of Gels (for 2 gels) Defreeze APS - Plug: Resolving gel (takes up 3/4 of plate) and after add a layer of butanol Wash of butanol with MilliQ before adding stacking gel (1/4 of plate). Acrylamide Plug Resolving gel Stacking gel 0.75 ml 2.5ml 0.5 ml Resolving gel (1M) Stacking gel mQW 10% SDS APS (40%) TEMED Plug x2 1.5 ml 3.75 ml Resolving gel x2 Stacking gel x2 5 ml 1 ml 7.5 ml 1.25 ml 1.25 ml 20 µl 1.25 µl 3.55 ml 3 ml 100 µl 50 µl 50 µl 25 µL 5 µL 2 µL 2.5 ml 2.5 ml 7.1 ml 6 ml 40 µl 200 µl 100 µl 100 µl 50 µl 2.5 µl 10 µl 4 µl III. Electrophoresis Electrophoresis Buffer: 28.82 g of Glycine 50 mL of Tris-HCl (pH 8.3) 20 mL of 10% SDS 2 L of mQW (can be recycled once) Put - Tris-HCl (PH=8.3): 100 mL of 1M Tris 39.8 mL of 1M HCl gels face inside! Leave protein samples in buffer for 15 min Boil the samples before loading in hot plate: 70 °C, 5 min Load 20µg in 15 µL of each diluted (in sample buffer) sample in each lane. Use 15 µL of the Pre-Stain molecular weight (high MW) markers Add 15 µL of straight sample buffer to empty lanes. Gel is run at 125V constant voltage for 60 min at room temperature. 1 Western Blotting for CB1 Aurélie, 2006 IV. Transfer (Western Blotting) Transfer Buffer: 100 mL of 10x Transfer Buffer 100 mL of Methanol 1 L with mQW PH: Add 2 Sodium Hydroxide Pellet (pH =11) - 10x Transfer Buffer: 22.13 g of CAPS 1 L with mQW PVDF membrane: 8.8 cm x 7.5 cm (don’t forget to mark it) Soak the membrane in 100% Methanol for 15 minutes to activate Soak filter paper, foam, and PVDF membrane in transfer buffer Black – Foam – Filter – Gel – Membrane – Filter – Foam – White Remove air bubbles Transfer for 120 min at 280mA constant current on ice. V. Blocking Blocking Solution: 4 g of Milk in 40 mL of Washing Buffer (for 2 Gels) - Membrane is left covered in blocking solution 1 hour (shaking at RT). VI. Antibody Incubation Antibody Incubation Buffer: 2 g of Milk in 40 mL of Washing Buffer (for 2 Gels) 10x Washing Buffer: 1 L of 1M Tris-HCl 175.3 g of NaCl 36.2 mL of Tween 20 2 L with mQW 1M Tris-HCl: 456.5 mL of 1M HCl 543.5 mL of 1M Tris 1M HCl: Stock at 11.8M 85 mL of Stock in 1 L - Rinse the membrane with antibody incubation buffer (AIB) once (4 mL). The primary antibody CB1 Rabbit 1/500 (30 µL) is added to PVDF soaked in 15 ml of the antibody incubation solution and left shaking at 4° overnight. SECOND DAY: Defreeze antibody II - 3x 5min and 4x quick washes in washing buffer, and rinse with AIB once (4 mL). The secondary antibody anti Rabbit 1/5 000 (3 µL) is added to PVDF soaked in 15ml of the antibody incubation buffer and left shaking at RT for 1 hour. 3x 5min and 4x quick washes in washing buffer, and a final wash in mQW. VII. Band analysis by Densitometry by Chemiluminescence (elkus_1807) - West Dura chemiluminescent substrate: 1mL A + 1mL B for 2 Blots - Exposure on the KODAK imaging station: 30-40 min - CB1: 60 kDA (3rd band of Marker) VIII. Stripping of the membrane - 5X Tris solution at 0.3152 M (38.1392 g/L), pH 6.8. Add 10g of SDS per 100ml of 5X Tris. - Dilute to 1X with mQW, and add 0.35 mL β-mercaptoEtOH per 50 mL of 1X Tris. - Incubate at 50°C for 30 min - 5 washes - Reblock the membrane if use of neurofilament 2 Western Blotting for CB1 Aurélie, 2006 IX. Proteins detection with SYPRO - Allow the membrane to dry completely - Float the mb face down in 7% acetic acid, 10% methanol for 15 min - 4 washes of 5 min with mQW - Incubate with SYPRO for 15 min (SYPRO can be re-utilized) - 2-3 washes of 1 min in mQW - Allow the membrane to dry completely - Imaging with UV at 590 nm (don’t forget to switch off UV after use) 3









