Human DNA Fingerprinting: Alu PCR
advertisement

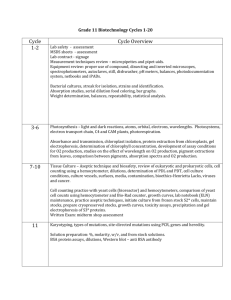
Human DNA Fingerprinting: Alu PCR Name(s) __________________, _________________ Each lab partner completes lab worksheet and retains for self study Background: In this experiment, polymerase chain reaction (PCR) is used to amplify (make more copies of) a short DNA sequence within chromosome #8. The short sequence is called Alu and appears within a gene called tissue plasminogen activator (TPA). Although the DNA from different individuals is more alike than different, there are many regions of the human chromosomes that exhibit a great deal of diversity. Such variable sequences are termed "polymorphic" (meaning many forms) and provide the basis for genetic disease diagnosis, forensic identification, and paternity testing. Location of TPA gene (8p12) Chromosome 8 The Alu family of short interspersed repeated DNA elements is distributed throughout primate genomes. Also known as “the jumping gene”, Alu reproduces by copying itself and inserting into new chromosomal locations. Over the past 65 million years, the Alu sequence has been copied over 1 million times and now makes up about 10% of the human genome (Cold Spring Harbor Laboratories, Cold Spring Harbor, NY). Within the last one million years Alu inserted itself within a gene called TPA-25. In the body, cells lining blood vessels will produce TPA (an enzyme) to dissolve a blood clot. This insertion is dimorphic, meaning that it is present in some individuals and not in others. PCR can be used to screen individuals for the presence (or absence) of the TPA-25 insertion. In this experiment, single stranded primers will attach to both sides of the TPA-25 gene and then make millions of copies. If the Alu sequence is present, a 400-bp fragment will be produced. When Alu is not present, a 100-bp fragment is produced. There are three possible genotypes that can be distinguished using gel electrophoresis: homozygotes for presence of TPA-25 homozygous for absence of TPA-25 heterozygotes A B (400-bp fragment only) Lane C (100-bp fragment only) Lane B (400-bp and 100-bp fragments) Lane D C D E lanes wells 400 bp 100 bp The source of template DNA are cells found in the root of human hairs. A solution called Chelex is used to extract the DNA from these cells. Then the cells are heated and boiled causing them to break open releasing the prized DNA molecules. After being spun in a microfuge, the supernatant containing genomic DNA is placed in a special microtube that contains a small white bead. This bead contains the replicating enzyme called Taq polymerase, the four deoxynucleotides (Adenine, Cytosine, Guanine, Thymine), and a cofactor called magnesium chloride which helps the enzyme Taq polymerase work. After the single stranded primers are added and some mineral oil to prevent the DNA from boiling over, the tube is placed in a temperature cycling machine called a thermocycler. This machine will heat up the DNA to 95 degrees causing it to split into two single strands. When the temperature is lowered to around 65 degrees, the primers border the sides of a targeted sequence of DNA. Finally the temperature is increased to 72 degrees allowing Taq polymerase to build the new DNA. All this happens in just 5 minutes. And after 30 cycles, over a million copies of the targeted sequence is produced. For more information on this process, read the diagram on page 4. TPA-25 locus on chromosome 8 Alu Maternal Upstream primers Downstream primers Paternal The "upstream" primer, "5-GTAAGAGTTCCGTAACAGGACAGCT-3", brackets one side of the TPA locus, while the "downstream" primer, "5-CCCCACCCTAGGAGAACTTCTCTTT-3" brackets the other side. The size of the amplification product(s) depends on the presence or absence of the Alu insertion at the TPA-25 locus on each copy of chromosome 8. In order to compare the genotypes from a number of different individuals, aliquots of the amplified sample and those of other experimenters are loaded into wells of an agarose gel--along with the DNA size markers and an unamplified control. Following electrophoresis and staining, amplification products appear as distinct bands in the gel--the distance moved from the well is inversely proportional to the presence or absence of the TPA-25 insertion. One or two bands are visible in each lane, indicating that an individual is either homozygous or heterozygous for the Alu insertion. Materials: 3 or 4 hairs with roots 1.5ml microtubes 10% Chelex solution with 50 ug/ml protease K 560C water bath Vortex Boiling water bath Timer Permanent marker Centrifuge ice DNA ladders Mineral oil Up and downstream primers Loading dye 2% agarose gel 1x TBE buffer Tips Staining trays forceps 20, 200 ul micropipettors Used tip beaker Paper ethidium bromide Disposable gloves Bioharzard bag I. Isolation of Genomic DNA from Hair 1. Obtain 3 to 4 hairs with root and sheath attached. Cut shafts of hair to approximately 2 cm lengths and put in 1.5 ml microtube. 2. Shake Chelex solution. While beads are still resuspended, remove 150 ul and add to hairs in microtube. Cellular components contain heavy metal ions can interfere with Taq polymerase in PCR reaction. Chelex binds these metal ions and any DNAases that will degrade genomic DNA. 3. Vortex. Make sure hairs are in the solution. 4. Incubate at 56 C for 5 minutes 5. Vortex. Make sure hairs are in the solution. 6. Incubate at 56 C for another 10 minutes. hair with 7. Vortex. Make sure hairs are still resuspended. root and 8. Incubate in boiling water for 10 minutes. This breaks the cell open. sheath 9. Vortex and then put on ice for 5 minutes. attached 10. Spin in microfuge for 5 minutes 11. Slowly and carefully remove tube. DO NOT disturb the pellet at bottom of tube. 12. Remove 75 ul of supernatant and place in a fresh tube. DO NOT transfer any chelex beads. 13. Label tubes and place on ice or store in freezer until ready to continue GOOD STOPPING POINT FOR SINGLE PERIOD CLASSES. II. PCR Reaction Set Up and Amplification 1. Use a permanent marker to label a PCR tube supplied by your teacher. It will contain a white bead that contains reagents for the amplification reaction. When the DNA is added to the tube, make sure the bead dissolves. 2. Use the matrix below as a checklist while added reagents to the PCR tube. Tube Hair Cell DNA Upstream primer Name 20 ul 2.5 ul Downstream primer 2.5 ul Total volume 25 ul 3. Add one drop of mineral oil by using a plastic pipette. Make sure not to touch the pipette to the PCR tube as it may contaminate other reactions with your preparation. 4. Close tube cap tightly and place in block of automated thermal cycler. Thermocycler settings: 30 cycles 94 C for 1 minute – this is when the double stranded DNA is denatured to single strands. 58 C for 2 minutes – this is when the primers will attach to specific DNA sequences of single strands. 72 C for 2 minutes – this is when Taq polymerase will add new bases to make the complementary strands . III. 2.0% Agarose Gel Preparation 1. Carefully pour agarose solution into the gel casting tray to fill to a depth of 5 mm. The gel should cover about one-third of the height of the comb teeth. 2. After the gel has solidified, remove it from the gel tray and place into a labeled staining tray. 3. Add 3-5 ml of 1xTBE buffer to keep gel most and cover with saran wrap or place into zip-lock bags and refrigerate until needed. GOOD STOPPING POINT FOR SINGLE PERIOD CLASSES. IV. Load Gel and Electrophorese. 1. Carefully transfer 2.0% agarose gel from staining tray back into gel tray. 2. Place gel tray into electrophoresis chamber making sure the wells are facing the negative pole. 3. Add enough electrophoresis buffer (1x TBE) to cover the gel. (approx. 350 ml). 4. Transfer 10 ul of the amplified product from the PCR reaction tube onto a clean square of parafilm. DO NOT transfer any mineral oil. This is best done by dipping pipet tip through mineral oil on top of PCR tube and carefully withdrawing 10 ul of amplified product. Before expelling onto parafilm, use a Kimwipe to remove any mineral oil clinging to outside of the pipet tip. 5. Add 1 ul of loading dye to the 10ul of your PCR sample and slowing mix the solution by pippetting up and down. (Hint: you will know if you have a clean prep because mineral oil will not mix with the loading dye. If free of the oil, your DNA sample mixes well with the dye). 6. Load 10 ul of your PCR reaction to a sample well in the gel. Note your lane position. 7. Add 10 ul of DNA ladders to specified wells in the diagram. DNA ladders Student samples 8. Electrophorese at 100 volts for about 30 minutes. Adequate separation has occurred when bromophenol blue bands have moved 30 mm from wells. 9. Turn off power supply, removing gel tray from electrophoresis chamber, and transfer gel to staining tray. 10. Follow protocol for staining with paper ethidium bromide. Hand In This Page Only Name(s) ___________________, _____________ V. Results and Discussion 1. After a photograph of your gel has been taken, orient the photograph with the sample wells at the top. 2. Attach photo in space provided and label the lanes 1 through 8, noting which lanes were designated for the ladders and ones for student samples. Attach Gel Photo Here 3. Using the DNA ladders as a guide, view the student sample lanes and note any allele bands. Note: it is possible that the primers may have been amplified themselves creating a primer dimmer. These are not products from the DNA and should be less than 100 bp in length. 4. Complete the data table below to determine genotype distribution of class. Student Samples Homozygous for Heterozygous for Homozygous – no the insert (+,+) the insert (+,-) insertion (-,-) 1 2 3 4 5 6 7 8 9 10 11 12 Totals 4. Calculate the allele frequency for the presence of the Alu insert in the population. Note: humans are diploid and the total number alleles present are 2 x the total population. So if there are 12 students in the population that means there are 24 total alleles. Show your work and circle your answers. 5. Calculate the allele frequency for the absence of the Alu insert in the population. Show your work and circle your answers 6. How could this data be utilized in a forensic investigation? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 7. If you were able to determine that there is a 50% chance (6 out of 12) that someone else in your school or city would have the same fingerprint as you, how does that percentage stand to identify one individual over another in a real forensic investigation? Comment on why you as a forensic scientist would use or not use the primers used in this lab procedure in a real forensic case? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 7. Is this individual or class evidence? Explain your answer. _____________________________________________________________________________________ _____________________________________________________________________________________ 8. Comment on the “general acceptance” of this scientific technique (Frye Standard). Would your scientific investigation hold up in a court of law if you were asked to take the stand? Please include a discussion of your training, the number of times you have done this technique, your sterile technique (food, gloves, sterilized lab bench), and laboratory procedures you followed (any possible mistakes made during procedure, identify possible steps in procedure that you may have accidentally contaminated your sample)? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________