Percent Composition Lab: Na2CO3 & NaCl Mixture
advertisement

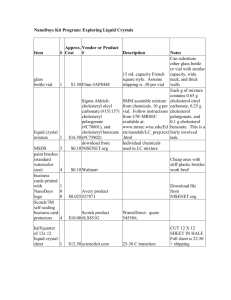
Percent Composition of a Solid Mixture (Guidelines: 1,2,3,5,6,7,9,11,12) Purpose: To use your knowledge of chemistry to determine the percent composition of sodium carbonate and table salt in a mixture of the two. Materials: 0.50 grams of a mixture of sodium carbonate and table salt, balance, 6.00 M HCl, 125 mL Erlenmeyer flask, water, graduated cylinders, vial, stopper with valve, tubing, pressure probe, forceps, temperature probe, ruler, brain (if you think of something else, let me know) Procedure: You decide. You must write this procedure so that someone else could perform the lab, using your description. (PERFORM TWO TRIALS). You must underline all of the materials in your procedure!!! Please write your procedure using bullets!!! (not a paragraph) Things to consider: Measure the volume of your gas by using water. What about the volume of the vial itself, the tubing, and the acid used? Will there be any other gas present? Yes, but… How much 6.00 M HCl will you need? (Include this in your calculations section!) Data: Make sure to record all relevant data. Calculations: Clearly show all calculations and equations needed to accomplish the purpose. Label calculations, include units, and circle relevant answers. (IF YOU USE THE SAME MASS OF SOLID AND THE SAME VOLUME OF HYDROCHLORIC ACID FOR EACH TRIAL, YOU CAN AVERAGE THE RESULT FROM THE TWO TRIALS TOGETHER.) Error Analysis: You will be given the actual composition of the mixture. Calculate your percent error. Discuss possible sources of error. Conclusion: Write a conclusion which demonstrates that you understand what you did. How did you accomplish the purposes? Discuss the math as well. Remember, here is a great guide for writing your conclusion: 1) Restate the purpose 2) Summarize the procedure and data collection 3) Discuss any important principles / equations used 4) Explain the graphs and/or math 5) Report your results Also, please include any other interesting information you would like to add.