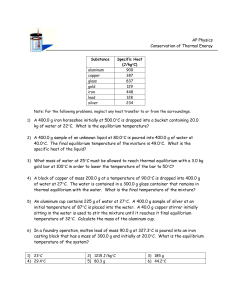
CHEMISTRY SCAVENGER HUNT
advertisement

CHEMISTRY SCAVENGER HUNT Remember, this is a team effort (teams no more than four). Find as many items as you can. Finding all 50 would be a tremendous feat!!! All samples should be in an appropriate container (solids in a ziploc bag and liquids in a plastic bottle), and each container should have attached to it a 3 inch by 5 inch index card with the following information: team members name, question #, answer to questions asked about the substance or required calculations. No substance can be used for more than one item on the scavenger list. The entire collection should be in a box decorated to represent your team and included the name of each member of your team. ITEMS FOR SCAVENGER HUNT Put a check mark next each item that you find in your hunt and place this sheet in your box of items. 1. 6.02 x 1023 of anything. Calculations required. 2. A cube made of sturdy material with a total volume of 1.0 L. On the note card include the dimensions of each side of the cube. Calculations required. 3. A cube made of sturdy material with a total volume of 1.0 mL. On the note card include the dimensions of each side of the cube. Calculations required. 4. An acid base indicator from nature other than one you have previously made for this class. The indicator can not be a substance sold as an indicator. State the source of the indicator, what color the indicator is with an acid and what color it is with a base. 5. A weak Arrhenius acid. Define Arrhenius acid. State the common and chemical names and chemical formula. 6. A weak Arrhenius base. Define Arrhenius base. State the common and chemical names and chemical formula. 7. A physical equilibrium system containing two or more phases. Define equilibrium. Identify the common name phase of the substances at equilibrium. 8. A chemical equilibrium system containing at least two phases. Define equilibrium. Write a balanced equation representing the equilibrium. 9. A chemistry cartoon cut from a newspaper or magazine. The cartoon from this handout is not acceptable. On the back of the note card include a brief statement about the concept that the cartoon is satirizing. 10. A 0.05 M solution of a substance. Include the calculations you did a description of how you made the solution, the common and chemical name of the compound, and chemical formula. 11. A heterogeneous mixture and a homogeneous mixture. Label each sample with the mixture it represents. Identify the ingredients in each mixture. 12. The mass of sodium chloride that would be produced when 2.3 g of sodium reacts with 0.56 L of chlorine gas. Assume STP and 100 % yield. Calculations and balanced equation required. 13. A list of all the winners of the Noble Prize in Chemistry for the last 10 years. Include what year they won the prize, the scientist(s) name(s), what country they are from, and a brief statement about the nature of their research. 14. A substance with a density greater than one. State the common name, chemical name and chemical formula of the substance. 15. Iron (III) oxide. Common name and chemical name required. 16. A homogeneous substance with exactly 29 protons in the nucleus of the atoms it is composed. Common name and chemical name required. 17. An inert gas. Common name and chemical name required. 18. A Bronsted Lowery Base. State the common name, chemical name and chemical formula for this substance. Define Bronsted-Lowery Base. 19. A product of an oxidation-reduction reaction. Chemical formula, common name and chemical name required. 20. An ionically bonded substance and a covalently bonded substance. Label each with the substance it represents. Chemical formulas, common names and chemical names required. 21. Dilute acetic acid. Common name and chemical formula required. 22. SiO2; Common name and chemical name required. 23. Polar molecules. Common name, chemical name, chemical formula required. 24. A metalloid. Common name, chemical name, chemical formula required. 25. Something to lower the freezing point of water. Common name, chemical name, chemical formula required. 26. A substance that dissolves endothermically. Common name, chemical name, chemical formula required. 27. A hydrocarbon with a molecular weight of > 100. Common name, chemical name, chemical formula required. 28. Nonpolar molecules. Common name, chemical name, chemical formula required. 29. An amount of water whose temperature would change by 15 oC when it absorbed 630 calories of heat energy. Calculations required. (no work, no credit, no kidding) 30. An equilibrium system containing carbonic acid, bicarbonate ions, and carbonate ions. Define equilibrium. Explain why, in terms of carbonic acid, bicarbonate ions, and carbonate ions, the system you are submitting is at equilibrium. 31. A substance that will change hydroxyapatite, Ca5(PO4)3OH, to fluorapatite, Ca5(PO4)3F. 32. A weak organic acid. Define weak organic acid. Common name, chemical name, chemical formula required. 33. Something containing titanium (IV) oxide. 34. An alkaline earth hydroxide. Chemical formula, common name and chemical name required. 35. A compound composed of an alkali metal and a halogen. Common name, chemical name, and chemical formula required. 36. A mixture which could be separated using paper chromatography. Define paper chromatography. Common name required. 37. Something containing a transition element from period 4. Identify the transition element on the back of the note card. 38. A nonmetal. Define nonmetal. Common name and chemical formula required. 39. An electrolyte. Define electrolyte. Common name, chemical name, chemical formula required. 40. Something containing L-carvone or D-carvone. Identify which one you have. What characteristic does it impart to the substance which distinguishes it from the item you did not bring in. 41. The solid product of the reaction between aqueous solutions of calcium chloride and sodium carbonate. Balanced equation, chemical formula and common name required. 42. An aqueous solution of a nonelectrolyte. Identify the solute and solvent by common name. 43. A molecule with bonding that follows the octet rule. Common name, chemical name, chemical formula required. 44. A hydrated crystal. Chemical formula, common name and chemical name required. 45. 2-propanol. Common name and chemical formula required. 46. 0.30 mol of sodium carbonate. Calculations, chemical formula and common name required. 47. A polymer of vinyl chloride. Define polymer. Identify the specific subunits of this polymer. Common name required. 48. An oxide of an element. Common name, chemical name, chemical formula required. 49. The salt produced when magnesium hydroxide reacts with sulfuric acid. Balanced equation, chemical formula, chemical name, and common name required. 50. The amount of NaCl produced from 10.0 g of sodium hydrogen carbonate reacting with an excess of hydrochloric acid. Assume 75 % yield. Balanced equation required. Scavenger Hunt Grading Criteria: Worth 10 points of BONUS. Grade will be determined by the number of items correctly identified and labeled. Every 5 items correctly identified and labeled will give you one point. To get credit for correctly answering a problem all calculations must be shown. The Team that finds the most items receives additional extra credit.