IB chemistry HL revision Option D
advertisement
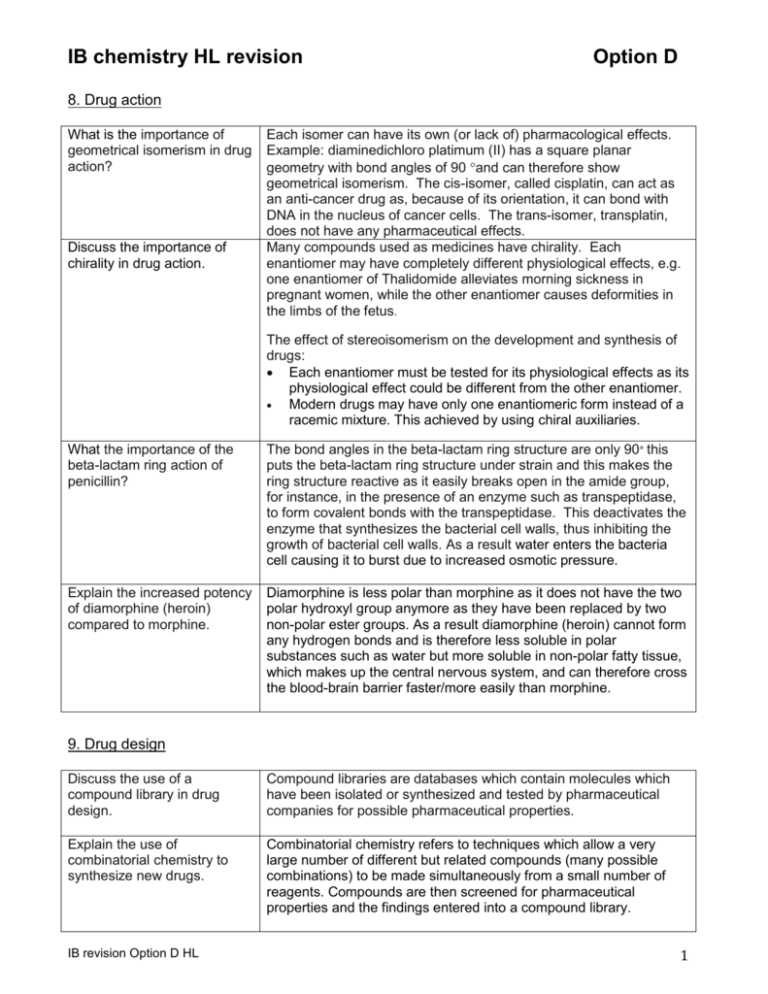
IB chemistry HL revision Option D 8. Drug action What is the importance of geometrical isomerism in drug action? Discuss the importance of chirality in drug action. Each isomer can have its own (or lack of) pharmacological effects. Example: diaminedichloro platimum (II) has a square planar geometry with bond angles of 90 and can therefore show geometrical isomerism. The cis-isomer, called cisplatin, can act as an anti-cancer drug as, because of its orientation, it can bond with DNA in the nucleus of cancer cells. The trans-isomer, transplatin, does not have any pharmaceutical effects. Many compounds used as medicines have chirality. Each enantiomer may have completely different physiological effects, e.g. one enantiomer of Thalidomide alleviates morning sickness in pregnant women, while the other enantiomer causes deformities in the limbs of the fetus. The effect of stereoisomerism on the development and synthesis of drugs: Each enantiomer must be tested for its physiological effects as its physiological effect could be different from the other enantiomer. Modern drugs may have only one enantiomeric form instead of a racemic mixture. This achieved by using chiral auxiliaries. What the importance of the beta-lactam ring action of penicillin? The bond angles in the beta-lactam ring structure are only 90° this puts the beta-lactam ring structure under strain and this makes the ring structure reactive as it easily breaks open in the amide group, for instance, in the presence of an enzyme such as transpeptidase, to form covalent bonds with the transpeptidase. This deactivates the enzyme that synthesizes the bacterial cell walls, thus inhibiting the growth of bacterial cell walls. As a result water enters the bacteria cell causing it to burst due to increased osmotic pressure. Explain the increased potency of diamorphine (heroin) compared to morphine. Diamorphine is less polar than morphine as it does not have the two polar hydroxyl group anymore as they have been replaced by two non-polar ester groups. As a result diamorphine (heroin) cannot form any hydrogen bonds and is therefore less soluble in polar substances such as water but more soluble in non-polar fatty tissue, which makes up the central nervous system, and can therefore cross the blood-brain barrier faster/more easily than morphine. 9. Drug design Discuss the use of a compound library in drug design. Compound libraries are databases which contain molecules which have been isolated or synthesized and tested by pharmaceutical companies for possible pharmaceutical properties. Explain the use of combinatorial chemistry to synthesize new drugs. Combinatorial chemistry refers to techniques which allow a very large number of different but related compounds (many possible combinations) to be made simultaneously from a small number of reagents. Compounds are then screened for pharmaceutical properties and the findings entered into a compound library. IB revision Option D HL 1 Explain the use of parallel chemistry to synthesize new drugs. The synthesis of a smaller but selected group of compounds in different reaction vessels; It involves producing a reactive intermediate bonded onto a resin bead which is then introduced into many different vessels producing a different compound in each vessel. What are the advantages of using solid-phase chemistry in drug synthesis? Reagents are adsorbed onto resin beads which allow better mixing of reagents to make all possible combinations. When synthesis reactions are complete, the products are removed easily from the beads by filtering off the beads and washing them. All reagents are mixed and then split into separate portions i.e. each portion has all reagents. To each portion a different reagent is added and a reaction is allowed to occur. The separate portions are then mixed again after which they are split into separate portion. To each portion a different reagent is added again… This is repeated Combinatorial libraries: databases used to search for molecules/groups with required/specific properties. Databases also include 3D images of molecules. 3D modeling software can be used to show interaction between medicine and active site on target molecule/receptor without actually making the medicine. This also allows them to design molecules with the perfect fit and then attempt to chemically produce them. Evaluation of (biological/pharmacological) effects of new drugs; if the structure of a new molecule is known the modeling software can then test its effectiveness in binding onto a receptor or enzyme. Equally, computers can be used to predict how changes to the structure of a drug might change its activity. It can do this by 3Dmodeling of the new shape or structure and use the model to find out how it interacts with target molecule. What is the split and mix technique? Describe how computers are used in drug design. Discuss how the polarity of a molecule can be modified to increase its aqueous solubility and how this facilitates its distribution around the body. Describe the use of chiral auxiliaries to form the desired enantiomer. IB revision Option D HL The polarity and therefore solubility in water can be increased by reacting molecules with acid or amine groups with a strong acid or alkali and change the molecules into ionic salts. The increased solubility also improves their uptake. Examples include aspirin which is reacted with sodium hydroxide to form an aspirin sodium salt and fluoxetine which is changed into fluoxetine hydrochloride. The chiral auxiliary attaches itself to the non-chiral molecule to force the reaction to follow a certain path i.e. the production of the desired enantiomer and not the other enantiomer. Once the new desired molecule has been formed, the auxiliary can be taken off and recycled. 2 10.Mind-altering drugs Describe the short term effects of lysergic acid diethylamide (LSD), mescaline, psilocybin. All three cause hallucinations i.e. hearing of voices and seeing of images which appear realistic but which do not exist i.e. there is no stimulus for them. The mind-altering drugs disrupt the activity of the brain transmitter serotonin that is involved in the processing of hearing and sight. Describe the effects typical to.. What functional groups are present in all three hallucinogenics LSD, mescaline and psilocybin? What are the structural differences between LSD, mescaline and psilocybin? What is the main ingredient of cannabis? What arguments can be used to support legalization of cannabis? What are arguments against the legalization? What are the effects of tetrahydrocannabinol? IB revision Option D HL LSD: psychological dependence, effects of LSD may be experienced a year or more later after last use i.e. “flashbacks” Mescaline: nausea, trembling, liver damage and reduced appetite Psilocybin: feelings of relaxation, develop tolerance, sense of well-being Benzene ring and amine groups. Use the data booklet to answer this!!! tetrahydrocannabinol no more or less damaging than other ‘legal’ drugs e.g. alcohol, tobacco offers pain relief from certain diseases such as AIDS and cancer relieves nausea in chemotherapy relieves tension and anxiety in terminally ill patients helps decrease pressure inside eyeball in patients suffering from glaucoma better control of quality of cannabis reduction of crime harmful effects: o may suppress immune system o respiratory ailments reduces ability to drive causes dependence the possibility of cannabis users moving on to harder drugs. loss of sense of time confusion light-headedness sense of floating loss of inhibitions euphoria mental relaxation 3








