RT-PCR Master Mix Prep
advertisement

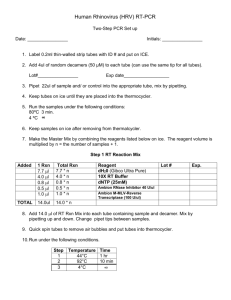
Human Bocavirus (HBoV) RT-PCR Two-Step PCR Set up Date: ________________ Initials: ________________ 1. Label 0.2ml thin-walled strip tubes with ID # and put on ICE. 2. Add 4ul of random decamers (50 μM) to each tube (can use the same tip for all tubes). Lot#________________ Exp date__________________ 3. Pipet 22ul of sample and/ or control into the appropriate tube, mix by pipetting. 4. Keep tubes on ice until they are placed into the thermocycler. 5. Run the samples under the following conditions: 80ºC 3 min. 4 ºC ∞ 6. Keep samples on ice after removing from thermalcycler. 7. Make the Master Mix by combining the reagents listed below on ice. The reagent volume is multiplied by n = the number of samples + 1. Step 1 RT Reaction Mix Added TOTAL 1 Rxn 7.7 l 4.0 l 0.8 l 0.5 l 1.0 l Total Rxn 7.7 * n 4.0 * n 0.8 * n 0.5 * n 1.0 * n 14.0ul 14.0 * n Reagent dH20 (Gibco Ultra Pure) 10X RT Buffer dNTP (25mM) Lot # Exp. Ambion RNase Inhibitor 40 U/ul Ambion M-MLV-Reverse Transcriptase (100 U/ul) 8. Add 14.0 l of RT Rxn Mix into each tube containing sample and decamer. Mix by pipetting up and down. Change pipet tips between samples. 9. Quick spin tubes to remove air bubbles and put tubes into thermocycler. 10. Run under the following conditions. Step 1 2 3 Temperature Time 44°C 1 hr 92°C 10 min 4°C ∞ Step Two PCR Date: ______________ Initials: ________________ 1. Label 0.2ml thin-walled strip tubes with ID # and put on ICE. 2. Calculate the appropriate amount of the PCR master mix by calculating n (n=the number of reactions and controls plus one. Multiply the single Rxn volume by this number. PCR Master Mix Added Total 1 Rxn 38.6 l 5.0 l 0.2 l 0.2 l 1.0 l 45.0 l 5.0 l x n = n Rxn 38.6 x n 5.0 x n 0.2 x n 0.2 x n 1.0 x n 45.0 X n 50.0 X n Reagent dH20 (Gibco Ultra Pure) 10X Pfu Buffer Lot # Exp. Forward primer HBoV01.2 [50mM] Reverse primer HBoV02.2 [50mM] Pfu DNA Polymerase [1.25 units] First strand cDNA 3. Assemble the PCR components on ice, and mix gently. 4. Aliquot 45ul of the PCR mix into the previously labeled 0.2ml strip tubes. 5. Using a multichannel pipet, pipet 5.0 l of the sample cDNA into the PCR mix and pipet up and down to mix. 6. Spin briefly and position tubes into the thermocycler. 7. Verify the program conditions before starting the program. Step 1 2 3 4 5 6 Date: ______________ Temperature 94°C 94°C 56°C 72°C 72°C 4°C Time 1 min 20 sec 20 sec 30 sec 5 min Return to Step# # Cycles 2 45 ∞ Initials: ________________ 1% Agarose Gel 100 ml 1X TAE Buffer 1 gram molecular grade agarose Bring to a boil. Cool to about 55oC. Add 1-2 µl EtBr. Stir well and pour into gel frame. Allow gel to solidify. Place solidified gel into a horizontal tank filled with 1X TAE. Load 7 µl ladder onto gel. Mix 2 µl 10X Blue Juice with 7 µl sample/ control. Load carefully onto gel. Run @ 120 V for approximately 2 hours. Start time_______________ Stop time_______________