Mass Spectrometry
DOI: 10.1002/anie.200((will be filled in by the editorial staff))
Generation of Gas-Phase Sodiated Arenes [(Na3(C6H4)+], and Other
Unusual Sodium Adducts Such As (Na3O)+, and (Na2OH)+
Athula B. Attygalle,* Chang-Ching Chan, Frank U. Axe and Mark S. Bolgar
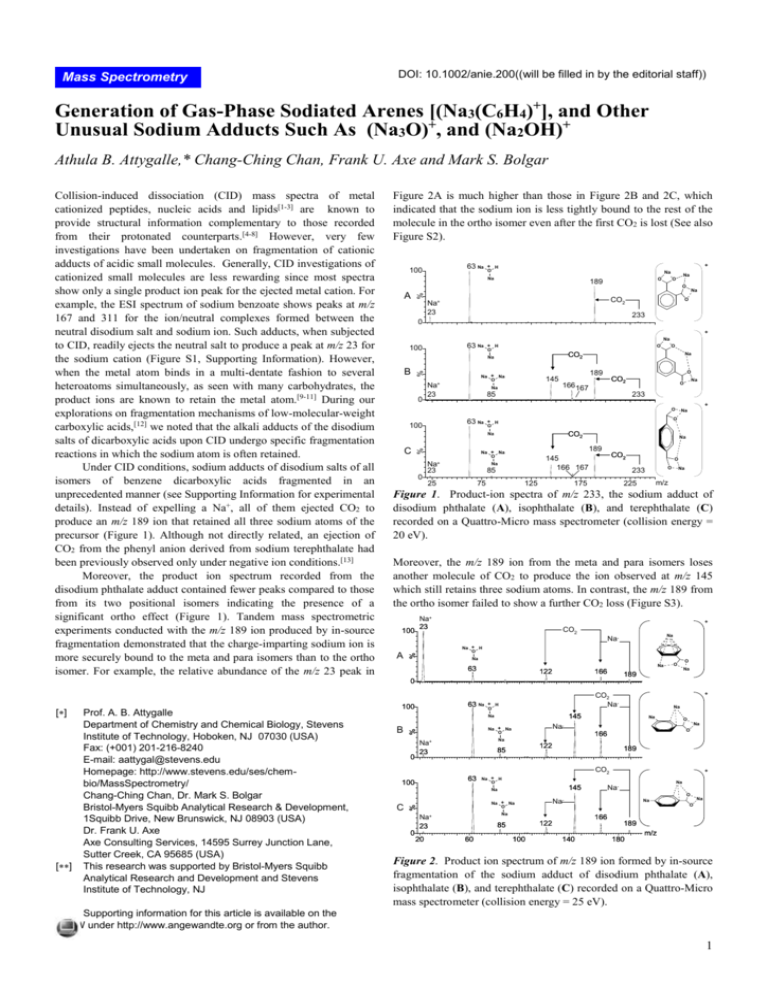
Figure 2A is much higher than those in Figure 2B and 2C, which
indicated that the sodium ion is less tightly bound to the rest of the
molecule in the ortho isomer even after the first CO2 is lost (See also
Figure S2).
63 Na
100
+
+ H
O
Na
Na
Na
O
O
189
O
%
A
CO2
Na+
23
Na
O
233
0
+
Na
63 Na
100
+ H
O
O
%
B
0
Na + Na
O
Na+
23
O
CO2
Na
Na
O
189
145
CO2
166 167
Na
85
233
+
100
O
CO2
%
0
85
50
75
CO2
145
166 167
Na
25
Na
189
Na + Na
O
Na+
23
Na
O
+ H
Na
C
Na
O
O
63 Na
100
125
150
175
O
O
233
200
225
Na
m/z
m/z
250
Figure 1. Product-ion spectra of m/z 233, the sodium adduct of
disodium phthalate (A), isophthalate (B), and terephthalate (C)
recorded on a Quattro-Micro mass spectrometer (collision energy =
20 eV).
Moreover, the m/z 189 ion from the meta and para isomers loses
another molecule of CO2 to produce the ion observed at m/z 145
which still retains three sodium atoms. In contrast, the m/z 189 from
the ortho isomer failed to show a further CO2 loss (Figure S3).
Na+
23
100
A
+
CO2
Na
Na.
Na + H
O
%
Collision-induced dissociation (CID) mass spectra of metal
cationized peptides, nucleic acids and lipids[1-3] are known to
provide structural information complementary to those recorded
from their protonated counterparts.[4-8] However, very few
investigations have been undertaken on fragmentation of cationic
adducts of acidic small molecules. Generally, CID investigations of
cationized small molecules are less rewarding since most spectra
show only a single product ion peak for the ejected metal cation. For
example, the ESI spectrum of sodium benzoate shows peaks at m/z
167 and 311 for the ion/neutral complexes formed between the
neutral disodium salt and sodium ion. Such adducts, when subjected
to CID, readily ejects the neutral salt to produce a peak at m/z 23 for
the sodium cation (Figure S1, Supporting Information). However,
when the metal atom binds in a multi-dentate fashion to several
heteroatoms simultaneously, as seen with many carbohydrates, the
product ions are known to retain the metal atom.[9-11] During our
explorations on fragmentation mechanisms of low-molecular-weight
carboxylic acids,[12] we noted that the alkali adducts of the disodium
salts of dicarboxylic acids upon CID undergo specific fragmentation
reactions in which the sodium atom is often retained.
Under CID conditions, sodium adducts of disodium salts of all
isomers of benzene dicarboxylic acids fragmented in an
unprecedented manner (see Supporting Information for experimental
details). Instead of expelling a Na+, all of them ejected CO2 to
produce an m/z 189 ion that retained all three sodium atoms of the
precursor (Figure 1). Although not directly related, an ejection of
CO2 from the phenyl anion derived from sodium terephthalate had
been previously observed only under negative ion conditions.[13]
Moreover, the product ion spectrum recorded from the
disodium phthalate adduct contained fewer peaks compared to those
from its two positional isomers indicating the presence of a
significant ortho effect (Figure 1). Tandem mass spectrometric
experiments conducted with the m/z 189 ion produced by in-source
fragmentation demonstrated that the charge-imparting sodium ion is
more securely bound to the meta and para isomers than to the ortho
isomer. For example, the relative abundance of the m/z 23 peak in
Na
Na
63
122
166
O
O
Na
189
0
[]
O
Na
O
Na.
Na
O
166
Na
Na+
23
0
Na
145
Na
B
+
CO2
Na.
+ H
Na + Na
O
%
Prof. A. B. Attygalle
Department of Chemistry and Chemical Biology, Stevens
Institute of Technology, Hoboken, NJ 07030 (USA)
Fax: (+001) 201-216-8240
E-mail: aattygal@stevens.edu
Homepage: http://www.stevens.edu/ses/chembio/MassSpectrometry/
Chang-Ching Chan, Dr. Mark S. Bolgar
Bristol-Myers Squibb Analytical Research & Development,
1Squibb Drive, New Brunswick, NJ 08903 (USA)
Dr. Frank U. Axe
Axe Consulting Services, 14595 Surrey Junction Lane,
Sutter Creek, CA 95685 (USA)
This research was supported by Bristol-Myers Squibb
Analytical Research and Development and Stevens
Institute of Technology, NJ
122
85
189
CO2
63
100
Na + H
O
C
20
40
60
80
100
120
140
Na
Na
O
166
122
85
O
Na.
Na
Na+
23
0
+
Na
Na.
145
Na
Na + Na
O
%
[]
63 Na
100
160
189
180
m/z
Figure 2. Product ion spectrum of m/z 189 ion formed by in-source
fragmentation of the sodium adduct of disodium phthalate (A),
isophthalate (B), and terephthalate (C) recorded on a Quattro-Micro
mass spectrometer (collision energy = 25 eV).
Supporting information for this article is available on the
WWW under http://www.angewandte.org or from the author.
1
In the optimized structure predicted by DFT calculations for
the m/z 233 ion from the ortho isomer (1), two of the sodium atoms
interact in a bidentate fashion with each carboxylate group, while
the third sodium atom bridges the two carboxylate groups (Scheme
1).
sodium atom bridges the two carboxylate groups. The bridging
interaction appears to distort the aromatic ring carbons attached to
the carboxylate group slightly out of plane particularly for the meta
and para isomers by 10° and 20°, respectively. In addition, the
bridging sodium atom particularly in the para isomer interacts with
the electrons of the benzene ring (6) (Scheme 3).
In contrast to that from the ortho isomer, the m/z 189 ions
derived from both meta and para-isomers undergo a second CO2 loss,
to produce a peak at m/z 145 indicating that the CO2 residue in the
m/z 189 ion derived from both meta and para-isomers is not as
securely bound as that of the ortho isomer’s. The optimized
structures computed for the m/z 189 ions from meta and para
isomers are shown on Schemes 2 and 3.
CO2
Scheme 1. Fragmentation of sodium adduct of disodium phthalate
m/z 189 [Na3C6H4(CO2)+] (7)
Once one of the carboxylate groups is eliminated, the sodium
atoms in the residual molecule are predicted to interact with the
main structural skeleton in three distinct ways (2). First, one of the
sodium atoms interacts in a bidentate fashion with the remaining
carboxylate group similar to that seen in the trisodium precursor.
Second, one of the sodium atoms is involved in a two-center twoelectron bond with the phenyl carbon, from which the carboxylate
group was eliminated, which also interacts with the remaining
carboxylate group as well. Third, the remaining sodium atom in the
ortho isomer interacts with all six carbon atoms of the phenyl ring in
a 6 fashion.[14] Since one of the oxygen atoms in the remaining
carboxylate group is also coordinated to the sodium atom bound to
the adjacent carbon atom of the benzene ring, the structure receives
considerable enhanced stability. Consequently, it is resistant to
further CO2 loss. In other words, upon CID the sodium ion that is
loosely coordinated by 6 cation-π interactions to the benzene ring
gets ejected with minimal collisional activation, rather than losing a
second CO2 molecule.
The coordination of sodium atoms in the precursor meta and
para isomers (3 and 6, respectively; Schemes 2 and 3) is somewhat
similar to that observed for the ortho isomer (1).
Scheme 2. Fragmentation of sodium adduct of disodium
isophthalate
For example, two of the sodium atoms in the sodium adducts
of disodium isophthalate (3) and terephthalate (6) interact in a
bidentate fashion with each carboxylate group, while the third
m/z 233 [Na3C6H4(CO2)2+] (6)
CO2
m/z 145 [Na3(C6H4)+] (8)
Scheme 3. Fragmentation of sodium adduct of disodium
terephthalate
In both structures 4 and 7, one sodium atom is bonded by a twocenter-two-electron bond to one of the phenyl ring carbons (in
contrast to that observed for the ortho isomer, this is an isolated
bond), one sodium atom interacts in a bidentate fashion with the
remaining carboxylate group, while the charge-bearing sodium atom
is simultaneously coordinated to electrons of the benzene ring and
one of the oxygen atoms of the remaining carboxylate group. In
other words, the charge-bearing sodium atom in structures 4 and 7
are more tightly coordinated to the main skeleton than that in
structure 2. Consequently, m/z 189 ion derived from the ortho
isomer (2) does not undergo a second CO2 loss, in contrast to those
from meta and para isomers which eliminate a second CO2 to
produce ions 5, and 8, respectively. The molecular formula of the
m/z 145 ion produced when a second CO2 is eliminated from meta
and para isomers is Na3C6H4+, which in fact represents the sodium
adduct of phenelenedisodium, an ionic species which has not been
reported previously. Although, aryl derivatives of the alkali metals
are known as useful strong bases and potent nucleophiles,[15] only on
one rare occasion has phenelenedisodium been mentioned in the
literature.[16] On the other hand, 1,4- and 1,3-dimetalations have
been accomplished with “spiny” arenes such as tert-butylbenzene.[17]
The observation of Baston et al[17] that 1,2-isomers of
dimetalloarenes are not formed at all, is congruent with our result of
not detecting a peak for Na3C6H4+ from ortho compounds. Moreover,
the m/z 145 sodium adducts we report here correspond to the lithium
and sodium salts of double-deprotonated benzene that have been
observed by Kass and his co-workers under negative ion
conditions.[13,18] Moreover, ortho ions have been observed to behave
different under negative ion conditions.[19]
To comprehend the unique fragmentations reported here, it is
necessary to understand the mechanism that allows retention of the
sodium atom originally coordinated to the carboxylate group when a
CO2 molecule is eliminated. For this elimination to occur, the
2
coordinating sodium ion must pirouette around the carboxylate
group and insert into the Caro-CO2 bond (Scheme 4). This process
was analyzed with DFT calculations for the sodium adduct of the
disodium salt of 1,4-benzene dicarboxylic acids (m/z 233) and found
to be reasonable, although all processes are energetically “up-hill”
(Figure S4).
On the other hand, in the m/z 189 ion from the ortho
compound (2), the inserted sodium atom ends up coordinating both
the aromatic carbon and the remaining carboxylate oxygen which
renders the second CO2 elimination much more difficult by this
mechanism.
mechanism for the ortho isomer is depicted in Scheme 6. In this
mechanism the bidentate coordinated sodium ion can swing around
the carboxylate group (red arrow) and insert into the C-CO2 bond
while displacing the bridging sodium ion into a -coordinating
position on the aromatic ring (blue arrow). Considering the
observation that a second CO2 loss was disfavored without the loss
of sodium radical, it is plausible that eliminating the 6 sodium ion
provides room for the remaining two sodium ions (2a) to rearrange
and allow the second CO2 elimination to occur.
+
+
Na
O
O
Na
Na
-CO2
O
O
Na
O
O
O
Scheme 4.
Fragmentation of sodium adduct of disodium
terephthalate (m/z 233).
Another intriguing observation that was made upon collision
activation was that all the three isomers of the m/z 189 ion expelled
a sodium radical to produce a peak at m/z 166 for a distonic radical
cation [(●C6H4CO2Na2)+] (Figure 2). However, the meta and para
isomers expelled the sodium radical to produce the m/z 166 radical
cation (10 and 11) more readily than the ortho isomer. Apparently,
the m/z 189 ion from the ortho isomers prefers to undergo a
heterolytic fragmentation to produce a sodium ion by eliminating a
neutral (12).
+
Na
Na
O
CO2
Na
C
Na2C6H4+
O
+
Na
O
C
m/z 122
Na
m/z 166 (ortho, 9)
(meta, 10)
(para, 11)
O
Na
O
m/z 189 (ortho, 2)
(meta, 4)
(para, 7)
C
+
Na
m/z 23
Na
O
Na
166 Da (12)
Scheme 5. Fragmentation of m/z 189 ion from ortho, meta and para
isomers.
In order to rationalize these differences, we calculated the
energetics of neutral sodium atom and sodium cation elimination
from isomeric m/z 189 ions (Scheme 5). The relative energies
obtained for these two competing processes demonstrate that the
elimination of the sodium atom directly bonded to phenyl ring as a
neutral atom is preferred for both meta and para isomers (4 and 7),
while for the ortho isomer elimination of the 6-bonded sodium
atom as a cation is favoured (Table 1).
m/z 189 (2)
m/z 166 (2a)
The m/z 166 radical cation [(●C6H4CO2Na2)+] for all three
isomers then eliminates CO2 to produce a peak at m/z 122
corresponding to the distonic cation (●C6H4Na2)+. The predicted
m/z 122 (2b)
Scheme 6. Fragmentation of m/z 189 derived from the sodium
adduct of disodium phthalate (m/z 233).
Also, our DFT calculations on both the m/z 166 and 122 ions
clearly predict that the unpaired electron resides primarily on the
coordinatively unsaturated aromatic carbon in all cases.
Two other unprecedented peaks in the spectra of sodium
adducts of disodium benzene dicarboxylates are those observed at
m/z 63 and 85 (Figure 1 and 3). The peak at m/z 63, which appeared
to represent sodiated sodium hydroxide, was particularly intriguing
since a proton is required for the formation of this charged species.
An abstraction of a proton from the aromatic nucleus of the benzene
dicarboxylate system is an energetically unfavorable process since
deprotonation energy of benzene to be about 402 kcal/mol. [20]
Moreover, the product ion spectrum of sodium adduct of disodium
[2,3,5,6-2H4]terephthalate showed no sign for a peak at m/z 64 for
[Na2OD]+, which confirmed that a proton is not extracted from the
aromatic system for the formation of this ion (Figure 3). In fact, the
proton originates from an unprecedented ion-molecule reaction that
has taken place with residual water in the collision cell. This was
confirmed by the observation of a peak exclusively at m/z 64 for
[Na2OD]+ (Figure S5) when product-ion spectra of m/z 233 ion were
recorded with D2O vapor introduced to the collision gas of the QToF tandem mass spectrometer. Since the relative abundance of the
m/z 63 peak was significantly higher in the tandem mass spectra
recorded from the m/z 189 ion produced by in-source fragmentation
of the meta- and para-isomers than that from the ortho isomers, it
appears that the m/z 189 ion structures proposed from the meta and
para-isomers show a higher affinity to water than that projected for
the ortho isomer.
149
100
+
O
193
63
%
D
Na
Na.
D
D
CO2
O
Na.
Na+
23
Na + Na
O
Na
85
0
Na
O
D
Na + H
O
Na
Table 1: Relative elimination energies calculated for ortho, meta,
and para isomers pertaining to the reaction, Na2C6H4(CO2)+ + Na•
→ Na2C6H4(CO2)• + Na+.
Isomer
E (kcal/mol)
ortho
-4.87
meta
+4.77
para
+3.81
Na
Na
Na
O
Na
Na
Na
O
O
Na
Na
Na
CO2
+
Na
25
50
75
100
CO2
O
Na
170
126
171
237
125
150
175
200
225
m/z
250
Figure 3. Product-ion spectrum of m/z 237 for the sodium adduct of
disodium [2,3,5,6-2H4]terephthalate recorded on a Quattro-Micro
mass spectrometer at a collision energy setting of 20 eV.
3
We propose that the initial ion/water complex (13) rapidly
rearranges into a transitional complex between NaOH and the
sodium adduct of sodium benzoate (15) (Scheme 7), which either
undergoes a direct dissociation to yield the ion observed at m/z 167
(which becomes m/z 168 if D2O is present in the collision gas), or
endure a transfer of the Na+ to NaOH to form the transitional
complex 16. Nicholas et al. have calculated that the energy barrier
between systems in which a sodium cation binds to either an oxygen
or a phenyl ring are very similar; hence the Na+ could be expected to
be mobile from one binding site to the another.[21] A subsequent,
separation of the constituents in the complex 16 leads to [Na2OH]+
(17) and sodium benzoate, or [Na3O]+ (18) and benzoic acid
(Scheme 7). It is interesting to note that compounds such as
Na3O(NO2) and Na3OBr have been reported previously.[22]
H
O
H
Na+
H
Na O
H
H
H
Na+
Keywords: radical ions · gas–phase chemistry · laser ablation · silver
· electron capture
[1] A. Favre, F. Gonnet, J.-C. Tabet, Int. J. Mass Spectrom. 1999, 190/191,
303.
[2] Y. Wang, J-S. Taylor, M. L. Gross, J. Am. Soc. Mass Spectrom. 2001,
O-Na+
Na O
Spectrom. 2003, 38, 87.
+
Na
[4] N. C. Polfer, J. Oomens, R. C. Dunbar, ChemPhysChem 2008, 9, 579.
m/z 85 (18)
O-Na+
O
O
(15)
(13)
[3] T. Aggerholm, S. C. Nanita, K. J. Koch, R. G. Cooks, J. Mass.
Na
Na O
Na
O
Received: ((will be filled in by the editorial staff))
Published online on ((will be filled in by the editorial staff))
12, 1174.
H
Na
documented.[12,23] Our results presented here demonstrate that such
reactions can be observed even in collision cells of tandem-in-space
instruments when the ions show very high affinity to water (for
example, ions bearing phenyl/metal bonds).
O-Na+
O
OH
[5] V. Sabareesh, P. Balaram, Rapid Comm. Mass Spectrom. 2006, 20, 618.
(16)
[6] X. Tang, W. Ens, K. G. Standing, J. B. Westmore, Anal. Chem. 1988, 60,
H
H
H
Na O
+
H
Na+
Na O
1791.
+
[7] D. Renner, G. Spiteller, Biomed. Environ. Mass Spectrom. 1988, 15, 75.
Na
O
O-Na+
m/z 63 (17)
O
O-Na+
m/z 167 (14)
Scheme 7. Proposed mechanism for the formation of m/z 63 and 85
ions
The reaction of water with highly basic ions bearing twocenter-two-electron bonds between a benzene nucleus and a metal
appears to be general phenomenon. For example, when the m/z 145
ion of formula Na3(C6H4)+ (which is the sodium adduct of
phenelenedisodium, obtained by two consecutive losses of CO 2
from the sodium adduct of disodium terephthalate) was isolated and
in an LTQ instrument at a normalized collision energy setting of 0,
and a relatively long storage time (300 ms), the product ions m/z 63,
m/z 85, and 123 were observed (Figure S6). The formation of these
ions from m/z 145 can be rationalized by mechanisms analogous to
those described in Scheme 7 for the m/z 189 ion. Thus, the peak
observed at m/z 123 (Figure S6), which in fact represents the sodium
adduct of phenyl sodium, originates from a rapid NaOH loss from
the water adduct.
Our results reveal that the ortho isomer of the disodium salts
of benzene dicarboxylic acids can be distinguished from those of the
meta and para isomers by the CID spectra of their sodium adducts.
Although the phenomenon is clearly an ortho effect, it is
dramatically different from the classical ortho effects observed
under electron ionization conditions in which a small molecule is
expelled from the molecular ions of some ortho compounds. In other
words, in the examples discussed here, the meta and para
compounds fragment differently, rather than the ortho compounds
undergoing a unique pathway. Complications arising from
unexpected ion-molecule reactions that take place particularly in ion
traps, due to inherent long ion residence times, have been well
[8] R. P. Grese, R. L. Cerny, M. L. Gross, J. Am. Chem. Soc. 1989, 111,
2835.
[9] C. Zhao, B. Xie, S.-Y. Chan, C. E. Costello, P. B. O'Connor, J. Am. Soc.
Mass Spectrom. 2008, 19, 138.
[10] K. M. Koshy, J.Y. Wang, J. M. Boggs, Biophys. J. 1999, 77, 306.
[11] M. Stano, H. D. Flosadottir, O. Ingolfsson, Rapid Comm. Mass
Spectrom. 2006, 20, 3498.
[12] A. B. Attygalle, N. Kharbatia, J. Bialecki, J. Ruzicka, A. Svatos, E. J.
Stauber, Rapid Comm. Mass Spectrom. 2006, 20, 2265.
[13] S. Bachrach, M. Hare, S. Kass, J. Am. Chem. Soc. 1998, 120, 12646.
[14] G. L. Miessler, D. Tarr, Inorganic Chemistry 3rd Ed., 2004, Pearson
Prentice Hall, Upper Saddle River, N.J. pp. 458.
[15] D. Seyferth, Organometallics 2006, 25, 2.
[16] A. A. Morton, C. E. Jr. Claff, J. Am. Chem. Soc. 1954, 76, 4935.
[17] E. Baston, R. Maggi, K. Friedrich, M. Schlosser, Eur. J. Org. Chem.
2001, 21, 3985.
[18] L. Pratt, S. R. Kass, J. Org. Chem. 2004, 69, 2123.
[19] D. R. Reed, M. Hare, S. R. Kass, J. Am. Chem. Soc. 2000, 122, 10689.
[20] G.E. Davico, V.M. Bierbaum, C.H. DePuy, G.B. Ellision, R.R. Squires,
J. Am. Chem. Soc. 1995,117, 2590-2599.
[21] J. B. Nicholas, B. P. Hay, J. Phys. Chem. A. 1999, 103, 9815.
[22] M. Janse, W. Z. f. Mueller, Z. Naturforsch. 1989, 44, 996.
4
Entry for the Table of Contents (Please choose one layout)
Layout 1:
Mass Spectrometry
A. Attygalle*, C.-C. Chan, F.U. Axe,
M.S. Bolgar.__________ Page – Page
2.61-2.66 Å
Generation of Gas-Phase Sodiated
Arenes [(Na3(C6H4)+], and Other
Unusual Sodium Adducts [(Na3O)+, and
(Na2OH)+]
Sticky Sodium: Unprecedented
sodiated arenes (see structure) can be
generated from sodiated disodium
terephthalate and isophthalate after two
successive expulsions of CO2
2.35 Å
Layout 2:
(Sticky Sodium)
2.61-2.66 Å
((Author(s), Corresponding Author(s)*))
__________ Page – Page
Generation of Gas-Phase Sodiated
Arenes [(Na3(C6H4)+], and Other
Unusual Sodium Adducts [(Na3O)+, and
(Na2OH)+]
2.35 Å
Sticky Sodium: Unprecedented sodiated arenes (see structure) can be generated
from sodiated disodium terephthalate and isophthalate after two successive
expulsions of CO2
5









